Abundant trans bonds make partially hydrogenated vegetable oil a very unhealthy food choice. Vegetable oil can also be hydrogenated until it becomes fully saturated with hydrogen atoms. Would the physical properties of the hydrogenated and partially hydrogenated oils differ? If so, how and why would the differences occur? Do you think that full hydrogenation makes vegetable oil more or less healthy to eat, or does it have no effect?
Lipids
The heterogeneous classes of organic compounds that are not water-soluble but are dissolved in organic solvents that are non-polar in nature are termed lipids. They are a long chain of fatty acids and esters of alcohols. Lipids are generally seen in several plants, microorganisms, and animals. They are utilized as insulation, components of the cell membrane, hormones, and molecules for the storage of energy.
Glycerophospholipid
Glycerophospholipid is the most abundantly occuring phospholipids found in the biological membranes. Lipids include a group of organic compounds like fats, hormones, oils, waxes, vitamins etc. They are non-polar molecules and are insoluble in water. Lipids play an important role in biological systems. They are the building blocks of our cell membranes, store energy and are involved in signaling.
Structure Of Camphor
A terpene with the molecular formula of C10H16O is a waxy, white color solid known as camphor. It is flammable. It also possesses a very pungent taste and a strong odor. There are various sources for extracting camphor from natural products such as the wood of the tree of camphor laurel. Sublimation of wood and steam distillation are some of the methods involved in obtaining camphor.
Glycolipid In Organic Chemistry
Glycolipids are lipids that are an important class of organic compounds in chemistry that have simple to complex applications. They contain carbohydrates, fatty acids, sphingolipids or a glycerol group. In other words, they are the modifications of lipids like acylglycerols, prenols and ceramides. They are all part of a wider group of compounds known as glycoconjugates.
Diterpenoid
The terpenoid class includes diterpenoids, which are chemical compounds with 20 carbon atoms. They are made up of four isoprene units and are derived from geranylgeraniol, a C20 precursor. They have a C20H32 basic structure. These characteristics distinguish diterpenoids from simple terpenes, which have just 10 carbon atoms.
1. Abundant trans bonds make partially hydrogenated vegetable oil a very unhealthy food choice. Vegetable oil can also be hydrogenated until it becomes fully saturated with hydrogen atoms. Would the physical properties of the hydrogenated and partially hydrogenated oils differ? If so, how and why would the differences occur? Do you think that full hydrogenation makes vegetable oil more or less healthy to eat, or does it have no effect?
2. Lipoprotein particles are relatively large, spherical clumps of protein and lipid molecules (see Figure 3.18) that circulate in the blood of mammals. They are like suitcases that move cholesterol, fatty acid remnants, triglycerides, and phospholipids from one place to another in the body. Given what you know about the insolubility of lipids in water, which of the four kinds of lipids would you predict to be on the outside of a lipoprotein clump, bathed in the water-based fluid portion of blood?
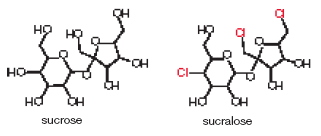
3. In 1976, a team of chemists in the United Kingdom was developing new insecticides by modifying sugars with chlorine (Cl2), phosgene (Cl2CO), and other toxic gases. One young member of the team misunderstood his verbal instructions to "test" a new molecule. He thought he had been told to "taste" it. Luckily for him, the molecule was not toxic, but it was very sweet. It became the food additive sucralose. Sucralose has three chlorine atoms substituted for three hydroxyl groups of sucrose (table sugar):
The altered sugar binds so strongly to the sweet-taste receptors on the tongue that the human brain perceives it as 600 times sweeter than sucrose. Sucralose was originally marketed as an artificial sweetener called Splenda®, but it is now available under several other brand names. Researchers investigated whether the body recognizes sucralose as a carbohydrate by feeding sucralose labeled with 14C to volunteers. Analysis of the radioactive molecules in the volunteers' urine and feces showed that 92.8 percent of the sucralose passed through the body without being altered. Some people are worried that the chlorine atoms impart toxicity to sucralose. How would you respond to that concern?
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images






