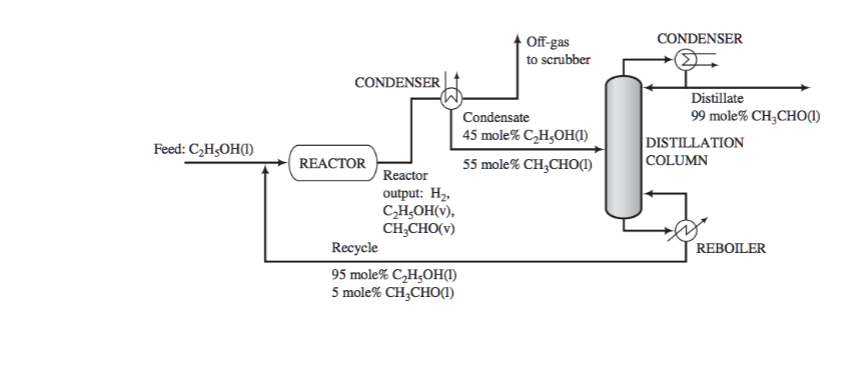
Acetaldehyde is synthesized by the catalytic dehydrogenation of ethanol: Fresh feed (pure ethanol) is blended with a recycle stream (95 mole% ethanol and 5% acetaldehyde), and the combined stream is heated and vaporized, entering the reactor at 280°C. Gases leaving the reactor are cooled to condense the acetaldehyde and unreacted ethanol. Off-gas from the condenser is sent to a scrubber, where the uncondensed organic compounds are removed and hydrogen is recovered as a by-product. The condensate from the condenser, which is 45 mole% ethanol, is sent to a distillation column that produces a distillate containing 99 mole% acetaldehyde and a bottoms product that constitutes the recycle blended with fresh feed to the process. The production rate of the distillate is 1000 kg/h. The pressure throughout the process may be taken as 1 atm absolute. If the distillate is at a temperature of 17.6 degrees C, what is the vapor pressure of acetaldehyde in this stream (atm)? Assume that the distillate is pure acetaldehyde
Acetaldehyde is synthesized by the catalytic dehydrogenation of ethanol:
Fresh feed (pure ethanol) is blended with a recycle stream (95 mole% ethanol and 5%
acetaldehyde), and the combined stream is heated and vaporized, entering the reactor at 280°C.
Gases leaving the reactor are cooled to condense the acetaldehyde and unreacted ethanol. Off-gas
from the condenser is sent to a scrubber, where the uncondensed organic compounds are
removed and hydrogen is recovered as a by-product. The condensate from the condenser, which
is 45 mole% ethanol, is sent to a distillation column that produces a distillate containing 99
mole% acetaldehyde and a bottoms product that constitutes the recycle blended with fresh feed
to the process. The production rate of the distillate is 1000 kg/h. The pressure throughout the
process may be taken as 1 atm absolute.
If the distillate is at a temperature of 17.6 degrees C, what is the vapor pressure of acetaldehyde in this
stream (atm)? Assume that the distillate is pure acetaldehyde.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps









