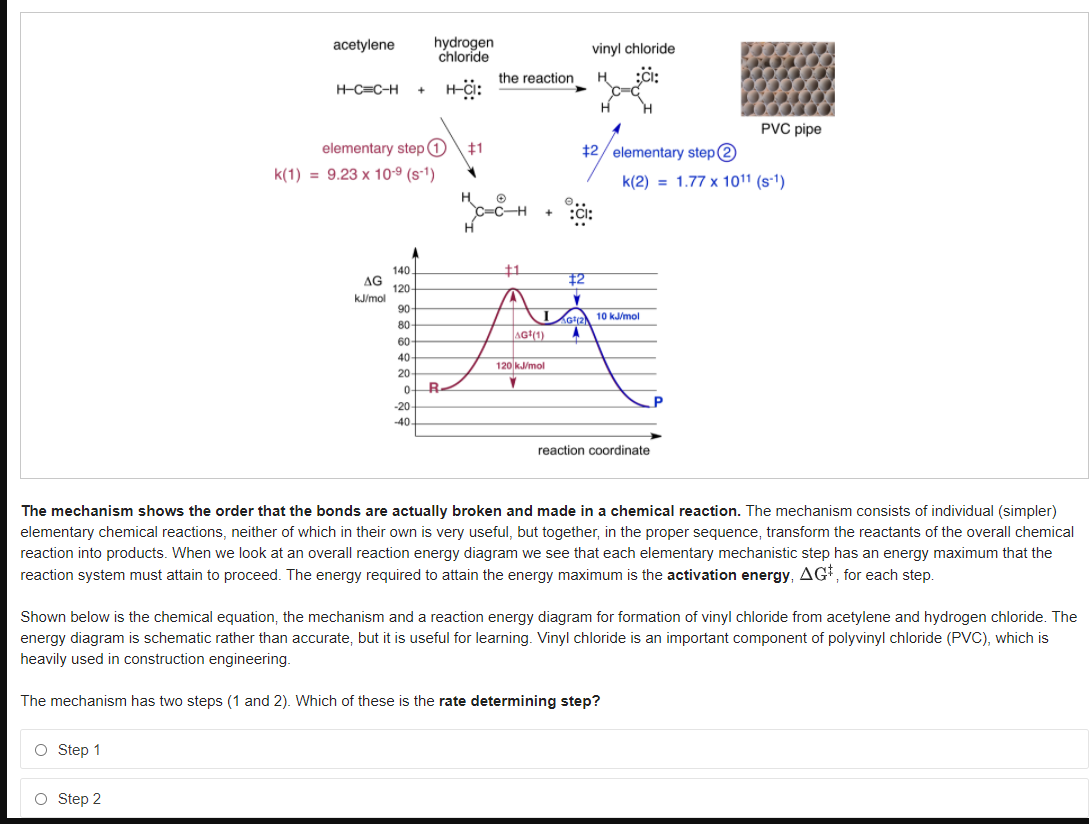
acetylene hydrogen chloride vinyl chloride the reaction H-C=C-H PVC pipe elementary step 0 #1 12/ elementary step 2 k(1) = 9.23 x 10-9 (s-1) k(2) = 1.77 x 1011 (s-') 140. 1 AG 120- 12 kJ/mol 90 10 kJ/mol 80 AG(1) 60 40 120 kJ/mol 20 0 R- P -20 -40 reaction coordinate The mechanism shows the order that the bonds are actually broken and made in a chemical reaction. The mechanism consists of individual (simpler) elementary chemical reactions, neither of which in their own is very useful, but together, in the proper sequence, transform the reactants of the overall chemical reaction into products. When we look at an overall reaction energy diagram we see that each elementary mechanistic step has an energy maximum that the reaction system must attain to proceed. The energy required to attain the energy maximum is the activation energy, AGI, for each step. Shown below is the chemical equation, the mechanism and a reaction energy diagram for formation of vinyl chloride from acetylene and hydrogen chloride. The energy diagram is schematic rather than accurate, but it is useful for learning. Vinyl chloride is an important component of polyvinyl chloride (PVC), which is heavily used in construction engineering. The mechanism has two steps (1 and 2). Which of these is the rate determining step? O Step 1 O Step 2
acetylene hydrogen chloride vinyl chloride the reaction H-C=C-H PVC pipe elementary step 0 #1 12/ elementary step 2 k(1) = 9.23 x 10-9 (s-1) k(2) = 1.77 x 1011 (s-') 140. 1 AG 120- 12 kJ/mol 90 10 kJ/mol 80 AG(1) 60 40 120 kJ/mol 20 0 R- P -20 -40 reaction coordinate The mechanism shows the order that the bonds are actually broken and made in a chemical reaction. The mechanism consists of individual (simpler) elementary chemical reactions, neither of which in their own is very useful, but together, in the proper sequence, transform the reactants of the overall chemical reaction into products. When we look at an overall reaction energy diagram we see that each elementary mechanistic step has an energy maximum that the reaction system must attain to proceed. The energy required to attain the energy maximum is the activation energy, AGI, for each step. Shown below is the chemical equation, the mechanism and a reaction energy diagram for formation of vinyl chloride from acetylene and hydrogen chloride. The energy diagram is schematic rather than accurate, but it is useful for learning. Vinyl chloride is an important component of polyvinyl chloride (PVC), which is heavily used in construction engineering. The mechanism has two steps (1 and 2). Which of these is the rate determining step? O Step 1 O Step 2
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter12: Kinetics
Section: Chapter Questions
Problem 11E: In the PhET Reactions under Options. (a) Leave the Initial Temperature at the default setting....
Related questions
Question
answer the following question
Transcribed Image Text:acetylene
hydrogen
chloride
vinyl chloride
the reaction
H-C=C-H
PVC pipe
elementary step 0
#1
12/ elementary step 2
k(1) = 9.23 x 10-9 (s-1)
k(2) = 1.77 x 1011 (s-')
140.
1
AG
120-
12
kJ/mol
90
10 kJ/mol
80
AG(1)
60
40
120 kJ/mol
20
0 R-
P
-20
-40
reaction coordinate
The mechanism shows the order that the bonds are actually broken and made in a chemical reaction. The mechanism consists of individual (simpler)
elementary chemical reactions, neither of which in their own is very useful, but together, in the proper sequence, transform the reactants of the overall chemical
reaction into products. When we look at an overall reaction energy diagram we see that each elementary mechanistic step has an energy maximum that the
reaction system must attain to proceed. The energy required to attain the energy maximum is the activation energy, AGI, for each step.
Shown below is the chemical equation, the mechanism and a reaction energy diagram for formation of vinyl chloride from acetylene and hydrogen chloride. The
energy diagram is schematic rather than accurate, but it is useful for learning. Vinyl chloride is an important component of polyvinyl chloride (PVC), which is
heavily used in construction engineering.
The mechanism has two steps (1 and 2). Which of these is the rate determining step?
O Step 1
O Step 2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning