After all that hard work, Thor and Captain America generate a laser with an output of 4.67 x 1019 J of energy. They try it out and burn a big hole in the Hulk's shorts..who subsequently trashes the place. What color laser did they end up making?
After all that hard work, Thor and Captain America generate a laser with an output of 4.67 x 1019 J of energy. They try it out and burn a big hole in the Hulk's shorts..who subsequently trashes the place. What color laser did they end up making?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter8: An Introduction To Optical Atomic Spectrometry
Section: Chapter Questions
Problem 8.10QAP: In high-temperature sources, sodium atoms emit a doublet with an average wavelength of 1139 nm. The...
Related questions
Question

Transcribed Image Text:After all that hard work, Thor and Captain America generate a laser with an output of 4.67 x 10 19 J of energy. They try it out and burn a big hole in the Hulk's
shorts..who subsequently trashes the place. What color laser did they end up making?
Consider these color ranges: blue 350-490 nm, green 490-570 nm, yellow 570-590 nm, orange 590-620 nm.
E-
h = 6.626 x 10-34 Js
C = 3 x 108 m/s
O red (651 nm)
O blue (425 nm)
O yellow (584 nm)
O green (536 nm)
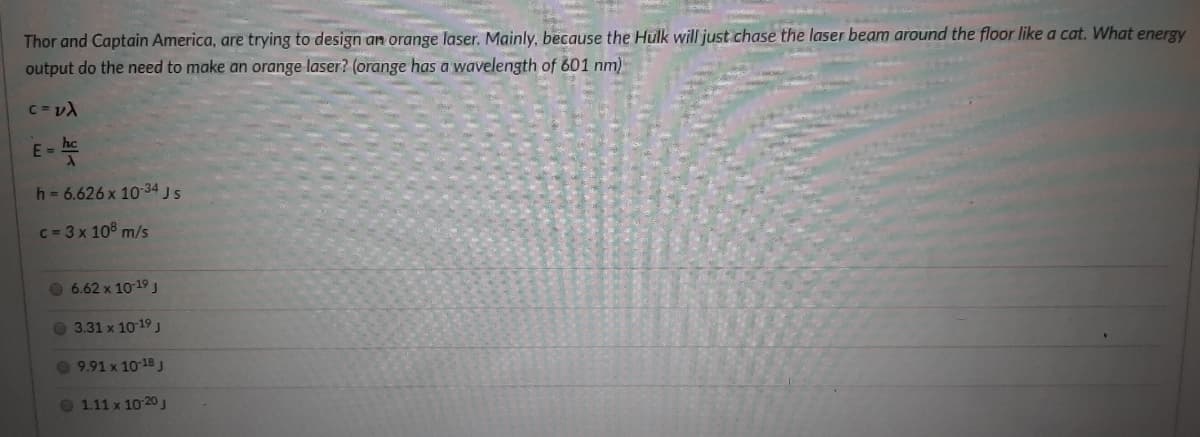
Transcribed Image Text:Thor and Captain America, are trying to design an orange laser. Mainly, because the Hulk will just chase the laser beam around the floor like a cat. What energy
output do the need to make an orange laser? (orange has a wavelength of 601 nm)
C = v)
E-
h= 6.626 x 10-34 Js
C= 3 x 108 m/s
6.62 x 10-19 J
O 3.31 x 1019
9.91 x 10 18 J
O 111 x 10 20J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning