After seeing Blake Lively's Met Gala 2022 look, a chemist was inspired to determine the copper concentration in their rock sample. Knowing that copper undergoes redox reactions, they chose to use an iodometric titration for this analysis. An unknown sample weighing 1.5098 g was acidified with 3.0 M H₂SO4 to dissolve the copper compounds and diluted with 30 mL distilled water. This was followed by the addition of excess KI producing I2 and Cul precipitate from Cu²+ (Reaction 1). Then, the liberated I2 reacted with the excess KI forming triiodide anion (Reaction 2), which was then titrated with Na2S2O3 titrant (Reaction 3). This entire solution required 17.60 mL of the titrant to reach the starch end point. MW: Cu (63.546 g/mol) a. Write the balanced chemical equation for Reactions 1-3 b. What is the mole ratio between Cu²+ and S203²-? Prior to the titration of the sample, the titrant was standardized by taking 50.0 mL of 6.7863 x 10-³ M standard KIO3 solution, acidifying, and adding KI crystals. Titration of this solution required 9.80mL to reach the starch endpoint. c. What is the concentration of the S2O3²- titrant in M? d. What is the copper content of the rock sample in %w/w?
After seeing Blake Lively's Met Gala 2022 look, a chemist was inspired to determine the copper concentration in their rock sample. Knowing that copper undergoes redox reactions, they chose to use an iodometric titration for this analysis. An unknown sample weighing 1.5098 g was acidified with 3.0 M H₂SO4 to dissolve the copper compounds and diluted with 30 mL distilled water. This was followed by the addition of excess KI producing I2 and Cul precipitate from Cu²+ (Reaction 1). Then, the liberated I2 reacted with the excess KI forming triiodide anion (Reaction 2), which was then titrated with Na2S2O3 titrant (Reaction 3). This entire solution required 17.60 mL of the titrant to reach the starch end point. MW: Cu (63.546 g/mol) a. Write the balanced chemical equation for Reactions 1-3 b. What is the mole ratio between Cu²+ and S203²-? Prior to the titration of the sample, the titrant was standardized by taking 50.0 mL of 6.7863 x 10-³ M standard KIO3 solution, acidifying, and adding KI crystals. Titration of this solution required 9.80mL to reach the starch endpoint. c. What is the concentration of the S2O3²- titrant in M? d. What is the copper content of the rock sample in %w/w?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter19: Principles Of Chemical Reactivity: Electron Transfer Reactions
Section19.9: Corrosion: Redox Reactions In The Environment
Problem 2.4ACP: The overall reaction for the production of Cu(OH)2 from Cu in oxygenated water can be broken into...
Related questions
Question
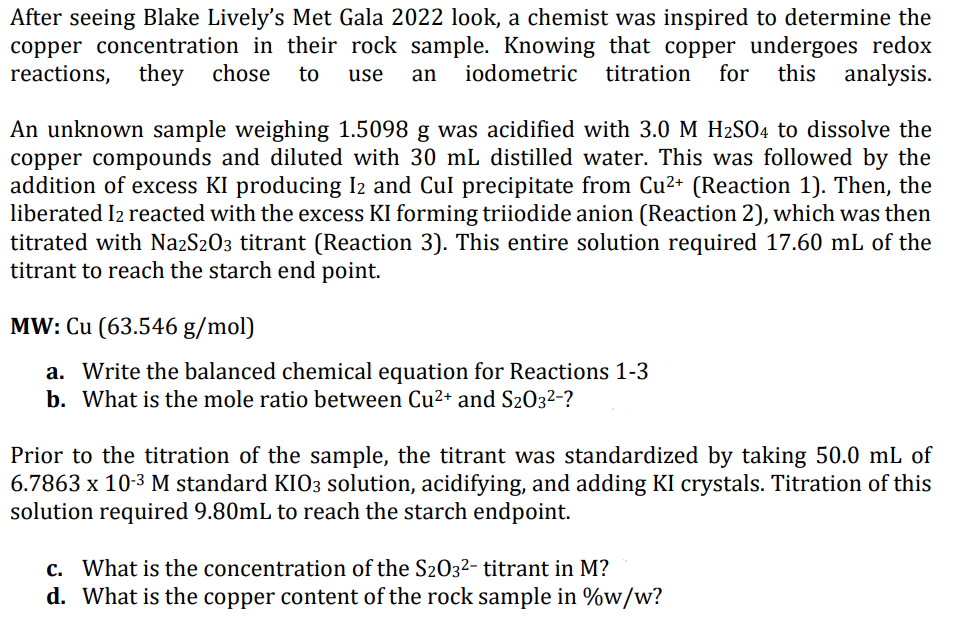
Transcribed Image Text:After seeing Blake Lively's Met Gala 2022 look, a chemist was inspired to determine the
copper concentration in their rock sample. Knowing that copper undergoes redox
reactions, they chose to use an iodometric titration for this analysis.
An unknown sample weighing 1.5098 g was acidified with 3.0 M H₂SO4 to dissolve the
copper compounds and diluted with 30 mL distilled water. This was followed by the
addition of excess KI producing I2 and Cul precipitate from Cu²+ (Reaction 1). Then, the
liberated I2 reacted with the excess KI forming triiodide anion (Reaction 2), which was then
titrated with Na2S2O3 titrant (Reaction 3). This entire solution required 17.60 mL of the
titrant to reach the starch end point.
MW: Cu (63.546 g/mol)
a. Write the balanced chemical equation for Reactions 1-3
b. What is the mole ratio between Cu²+ and S203²-?
Prior to the titration of the sample, the titrant was standardized by taking 50.0 mL of
6.7863 x 10-³ M standard KIO3 solution, acidifying, and adding KI crystals. Titration of this
solution required 9.80mL to reach the starch endpoint.
c. What is the concentration of the S203²- titrant in M?
d. What is the copper content of the rock sample in %w/w?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
