After taking a mass spectrum on an unknown compound you found in the lab, you determined that the molecular formula was C7H8. Calculate the degrees of unsaturation for this unknown.
After taking a mass spectrum on an unknown compound you found in the lab, you determined that the molecular formula was C7H8. Calculate the degrees of unsaturation for this unknown.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter14: Mass Spectrometry
Section: Chapter Questions
Problem 14.27P: Following is the mass spectrum of an unknown compound. The two highest peaks are at m/z 120 and 122....
Related questions
Question
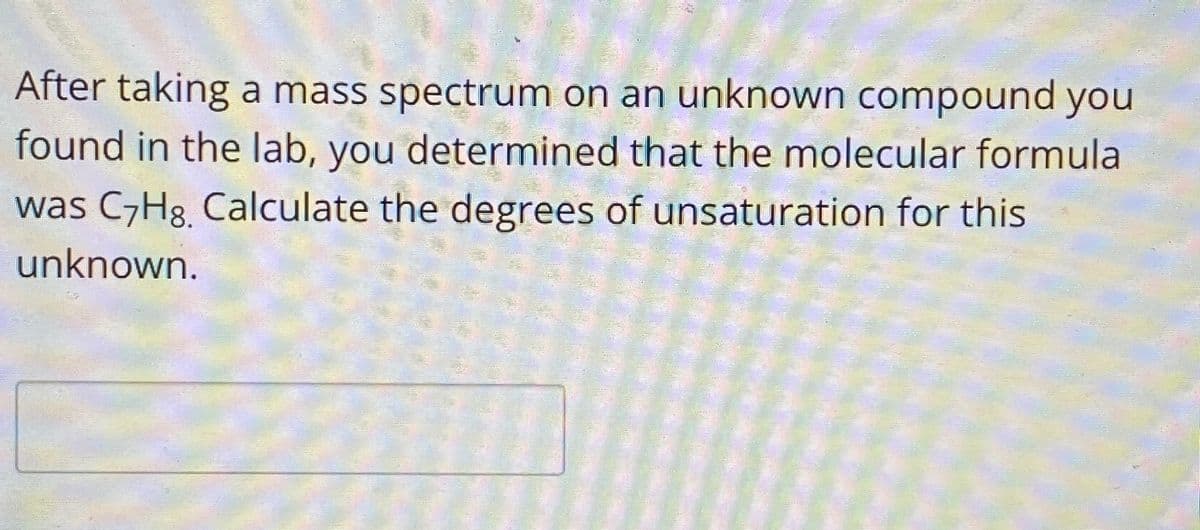
Transcribed Image Text:After taking a mass spectrum on an unknown compound you
found in the lab, you determined that the molecular formula
was C7H8. Calculate the degrees of unsaturation for this
unknown.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
