What would distinguish the mass spectrum of 2,2-dimethylpropane from those of 2 methylbutane and n-pentane? A. molecular ion peak at 72 B. base peak at 15 C. base peak at 27 D. base peak at 43 E. base peak at 57
What would distinguish the mass spectrum of 2,2-dimethylpropane from those of 2 methylbutane and n-pentane? A. molecular ion peak at 72 B. base peak at 15 C. base peak at 27 D. base peak at 43 E. base peak at 57
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter14: Mass Spectrometry
Section: Chapter Questions
Problem 14.27P: Following is the mass spectrum of an unknown compound. The two highest peaks are at m/z 120 and 122....
Related questions
Question
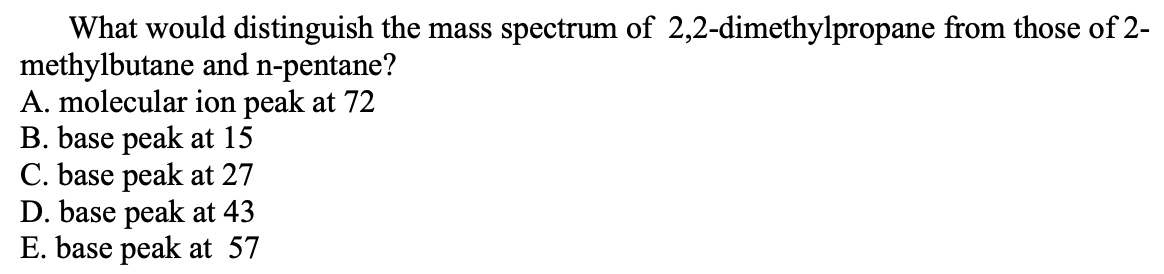
Transcribed Image Text:What would distinguish the mass spectrum of 2,2-dimethylpropane from those of 2-
methylbutane and n-pentane?
A. molecular ion peak at 72
B. base peak at 15
C. base peak at 27
D. base peak at 43
E. base peak at 57
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
