After taking the time to think about it, Wile E. Coyote's latest plan is to leave a trail of gasoline leading to the Roadrunner's house, and light the trail on fire. What could possibly go wrong! One of the components in gasoline is Heptane (C,H16), which burns according to the following balanced equation: C,H16(1) + 1102(g) → 7CO2(g) + 8H2O(g) Part A If the reaction as written has A.H° = -4464.5 kJ - mol-1, determine the enthalpy of formation for CH16(1). A¢H® Compound (kJ - mol-1) CO2(8) -393.5 H2O(g) -241.8 Express your answer in kilojoules per mole to one decimal place. AçH® = kJ mo]¬1
After taking the time to think about it, Wile E. Coyote's latest plan is to leave a trail of gasoline leading to the Roadrunner's house, and light the trail on fire. What could possibly go wrong! One of the components in gasoline is Heptane (C,H16), which burns according to the following balanced equation: C,H16(1) + 1102(g) → 7CO2(g) + 8H2O(g) Part A If the reaction as written has A.H° = -4464.5 kJ - mol-1, determine the enthalpy of formation for CH16(1). A¢H® Compound (kJ - mol-1) CO2(8) -393.5 H2O(g) -241.8 Express your answer in kilojoules per mole to one decimal place. AçH® = kJ mo]¬1
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 33PS: Nitrogen monoxide, a gas recently found to be involved in a wide range of biological processes,...
Related questions
Question
6
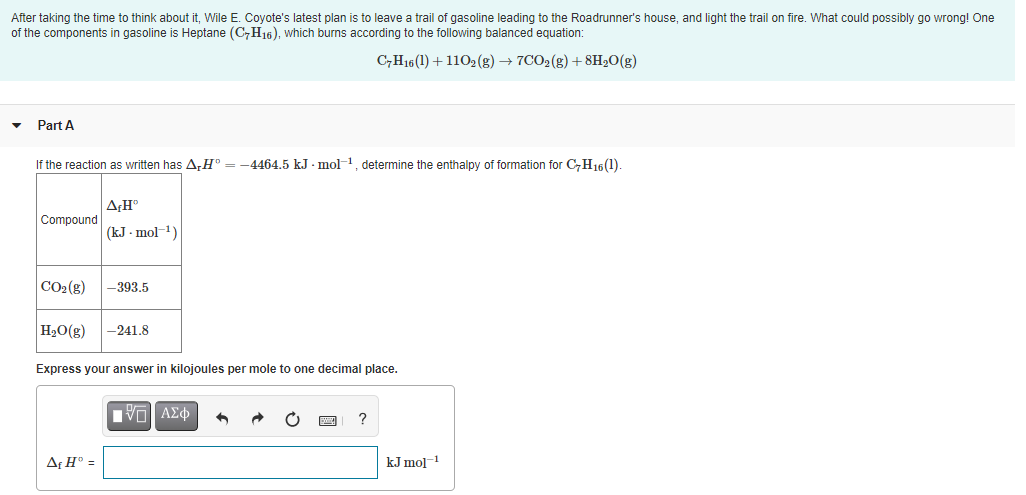
Transcribed Image Text:After taking the time to think about it, Wile E. Coyote's latest plan is to leave a trail of gasoline leading to the Roadrunner's house, and light the trail on fire. What could possibly go wrong! One
of the components in gasoline is Heptane (C,H16), which burns according to the following balanced equation:
C,H16(1) + 1102(g) → 7CO2(g) + 8H,0(g)
Part A
If the reaction as written has A,H° =-4464.5 kJ - mol-1, determine the enthalpy of formation for C,H16(1).
ΔΗ
Compound
(kJ - mol-1)
CO2(g)
-393.5
H2O(g)
-241.8
Express your answer in kilojoules per mole to one decimal place.
VO AZ
Af H° =
kJ mol1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning