Using the balanced equation for the synthesis of acrylonitrile (below), determine the amount of acrylonitrile that can be produced under the following conditions and the percent yield for this reaction. 2C3H6(g) + 2NH3(g) + 302(g) → 2C;H;N(g) + 6H20(g) 4. NEXT > The local chemical company makes a batch of acrylonitrile using 1.00 kg of propylene (C3H6, MW 42.08 g/mol), 1.50 kg of ammonia (NH, MW 17.03 g/mol) and 2.00 kg of oxygen (O2, MW 32.00 g/mol). Set up the table below that represents 100% yield with the given reaction conditions. Ignore the water side product. 2C3H6(g) 2NH3(g) 302(g) 2C,H;N(g) Before (mol) Change (mol) After (mol)
Using the balanced equation for the synthesis of acrylonitrile (below), determine the amount of acrylonitrile that can be produced under the following conditions and the percent yield for this reaction. 2C3H6(g) + 2NH3(g) + 302(g) → 2C;H;N(g) + 6H20(g) 4. NEXT > The local chemical company makes a batch of acrylonitrile using 1.00 kg of propylene (C3H6, MW 42.08 g/mol), 1.50 kg of ammonia (NH, MW 17.03 g/mol) and 2.00 kg of oxygen (O2, MW 32.00 g/mol). Set up the table below that represents 100% yield with the given reaction conditions. Ignore the water side product. 2C3H6(g) 2NH3(g) 302(g) 2C,H;N(g) Before (mol) Change (mol) After (mol)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section: Chapter Questions
Problem 89QRT
Related questions
Question
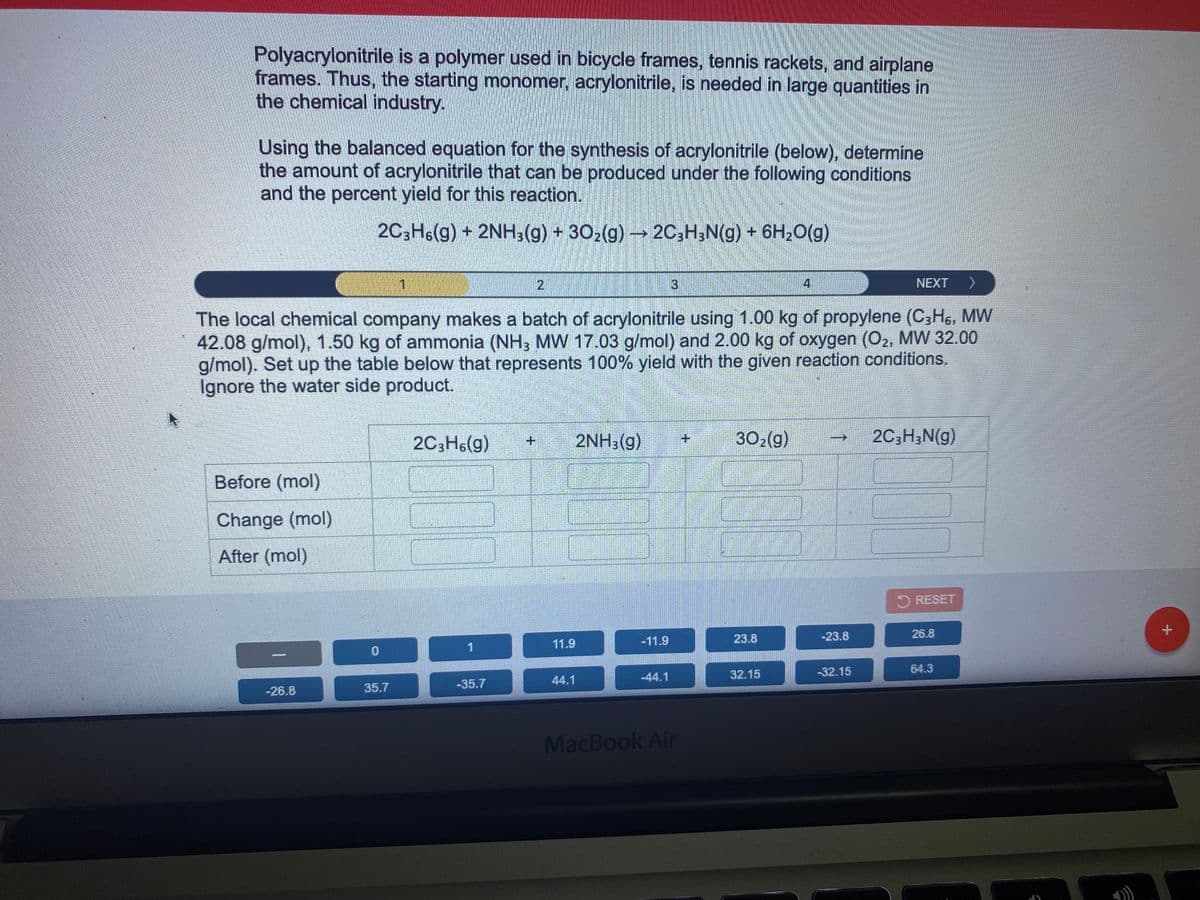
Transcribed Image Text:Polyacrylonitrile is a polymer used in bicycle frames, tennis rackets, and airplane
frames. Thus, the starting monomer, acrylonitrile, is needed in large quantities in
the chemical industry.
Using the balanced equation for the synthesis of acrylonitrile (below), determine
the amount of acrylonitrile that can be produced under the following conditions
and the percent yield for this reaction.
20,H6(g) + 2NH3(g) + 302(g) 2C,H,N(g) + 6H2O(g)
2,
3
4.
NEXT
>
The local chemical company makes a batch of acrylonitrile using 1.00 kg of propylene (C3H,, MW
42.08 g/mol), 1.50 kg of ammonia (NH3 MW 17.03 g/mol) and 2.00 kg of oxygen (O2, MW 32.00
g/mol). Set up the table below that represents 100% yield with the given reaction conditions.
Ignore the water side product.
2C3H6(g)
2NH3(g)
302(g)
2C;H;N(g)
Before (mol)
Change (mol)
After (mol)
5 RESET
-11.9
23.8
-23.8
26.8
1
11.9
-32.15
64.3
-44.1
32.15
-35.7
44.1
-26.8
35.7
MacBook Air
+.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div