Air is a mixture of gases that is about 78.0% N2 by volume. When air is at standard pressure and 25.0 °C, the N2 component will dissolve in water with a solubility of 4.88 × 10¬4 M. What is the value of Henry's law constant for N2 under these conditions? Express your answer with the appropriate units. Enter the unit M using the compound form mol/L. • View Available Hint(s) k = 6.26 • 10-4 M Submit Previous Answers X Incorrect; Try Again; 3 attempts remaining Enter your answer using units of Henry's law constant units.
Air is a mixture of gases that is about 78.0% N2 by volume. When air is at standard pressure and 25.0 °C, the N2 component will dissolve in water with a solubility of 4.88 × 10¬4 M. What is the value of Henry's law constant for N2 under these conditions? Express your answer with the appropriate units. Enter the unit M using the compound form mol/L. • View Available Hint(s) k = 6.26 • 10-4 M Submit Previous Answers X Incorrect; Try Again; 3 attempts remaining Enter your answer using units of Henry's law constant units.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 127QRT
Related questions
Question
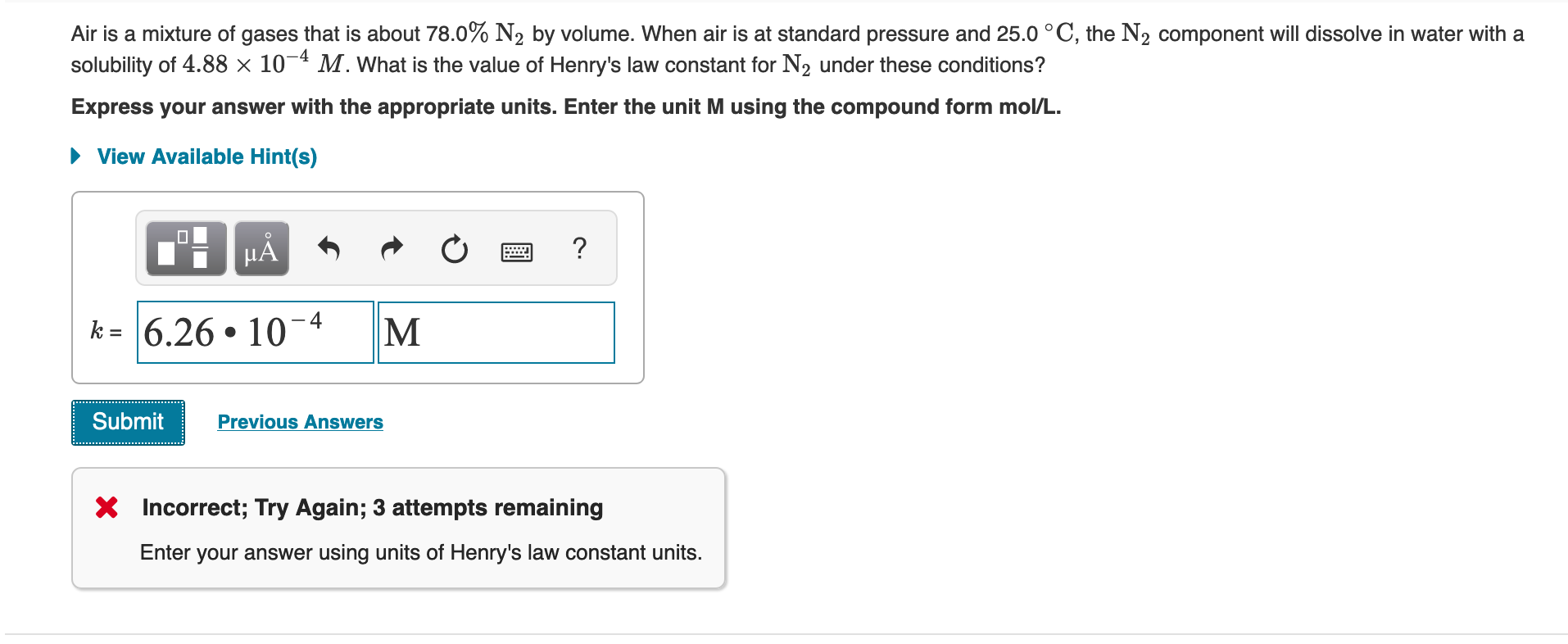
Transcribed Image Text:Air is a mixture of gases that is about 78.0% N2 by volume. When air is at standard pressure and 25.0 °C, the N2 component will dissolve in water with a
solubility of 4.88 × 10¬4 M. What is the value of Henry's law constant for N2 under these conditions?
Express your answer with the appropriate units. Enter the unit M using the compound form mol/L.
• View Available Hint(s)
k = 6.26 • 10-4
M
Submit
Previous Answers
X Incorrect; Try Again; 3 attempts remaining
Enter your answer using units of Henry's law constant units.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning