Allison dissolves as much as possible of a certain solid in a quantity of water at room temperature; she pours some of the solution into a evaporating dish, of known mass, and finds the combined mass of the dish and solution. After she evaporates the water, she finds the mass of the dish and solid. Her data is shown in the table below. Allison's Data Substance mass (in g) Dish 23.2 Dish and solution 34.3 Dish and solid 26.0 Using her data, Allison could use which of the following to find the solubility of the solid substance in g/100 cm3? O A 26.0 – 23.2 x 100 34.3 – 26.0 O B. 34.2 - 23.2 x 100 26.0 O C: 34.3 x100 26.0 – 23.2 O D. 34.3 - 23.2 x 100 26.0 – 23.2
Allison dissolves as much as possible of a certain solid in a quantity of water at room temperature; she pours some of the solution into a evaporating dish, of known mass, and finds the combined mass of the dish and solution. After she evaporates the water, she finds the mass of the dish and solid. Her data is shown in the table below. Allison's Data Substance mass (in g) Dish 23.2 Dish and solution 34.3 Dish and solid 26.0 Using her data, Allison could use which of the following to find the solubility of the solid substance in g/100 cm3? O A 26.0 – 23.2 x 100 34.3 – 26.0 O B. 34.2 - 23.2 x 100 26.0 O C: 34.3 x100 26.0 – 23.2 O D. 34.3 - 23.2 x 100 26.0 – 23.2
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 128IP: Some nonelectrolyte solute (molar mass = 142 g/mol) was dissolved in 150. mL of a solvent (density =...
Related questions
Question
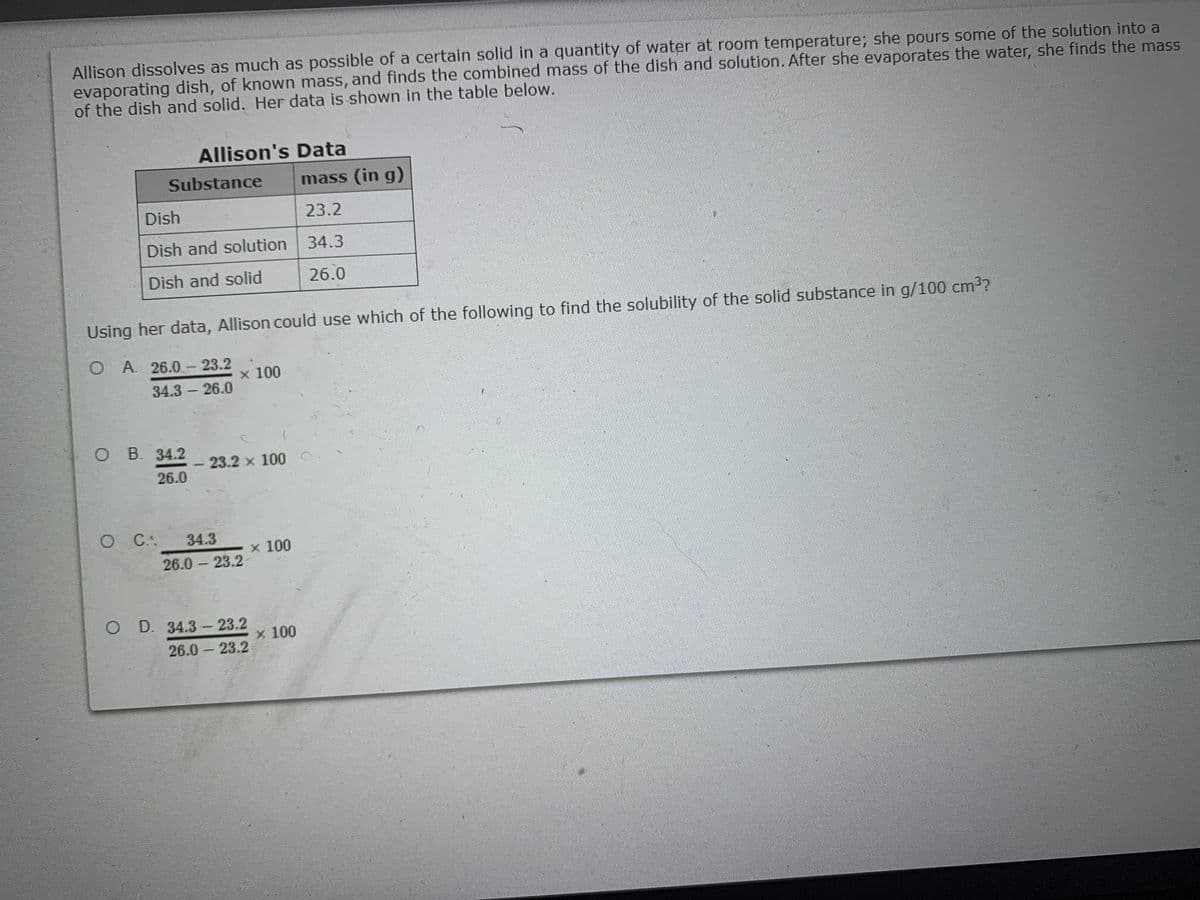
Transcribed Image Text:Allison dissolves as much as possible of a certain solid in a quantity of water at room temperature; she pours some of the solution into a
evaporating dish, of known mass, and finds the combined mass of the dish and solution.After she evaporates the water, she finds the mass
of the dish and solid. Her data is shown in the table below.
Allison's Data
Substance
mass (in g)
Dish
23.2
Dish and solution 34.3
Dish and solid
26.0
Using her data, Allison could use which of the following to find the solubility of the solid substance in g/100 cm3?
OA. 26.0- 23.2
x 100
34.3 26.0
-
O B. 34.2
23.2 x 100
26.0
OC:
34.3
x 100
26.0 23.2
O D. 34.3- 23.2
|
x 100
26.0-- 23.2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co
