Although p-hydroxybenzoic acid is less acidic than benzoic acid, o-hydroxybenzoic acid is slightly more acidic than benzoic acid. Explain this result. OH но -COOH -соон p-hydroxybenzoic acid o-hydroxybenzoic acid
Although p-hydroxybenzoic acid is less acidic than benzoic acid, o-hydroxybenzoic acid is slightly more acidic than benzoic acid. Explain this result. OH но -COOH -соон p-hydroxybenzoic acid o-hydroxybenzoic acid
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter23: Amines
Section: Chapter Questions
Problem 23.29P
Related questions
Question
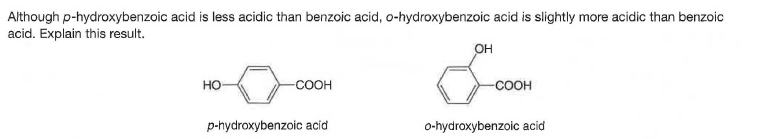
Transcribed Image Text:Although p-hydroxybenzoic acid is less acidic than benzoic acid, o-hydroxybenzoic acid is slightly more acidic than benzoic
acid. Explain this result.
OH
но
-COOH
-соон
p-hydroxybenzoic acid
o-hydroxybenzoic acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning