Ammonia can also be synthesized by the reaction: 3H2 (g) + N2(g)→2NH3(g) What is the theoretical yield of ammonia, in kilograms, that we can synthesize from 5.37 kg of H, and 34.5 kg of N2? Ηνα ΑΣφ theoretical yield of NH3 = 2.96 kg
Ammonia can also be synthesized by the reaction: 3H2 (g) + N2(g)→2NH3(g) What is the theoretical yield of ammonia, in kilograms, that we can synthesize from 5.37 kg of H, and 34.5 kg of N2? Ηνα ΑΣφ theoretical yield of NH3 = 2.96 kg
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.80PAE: 4.80 The reaction shown below is used to destroy Freon-12 (CF2Cl2), preventing its release into the...
Related questions
Question
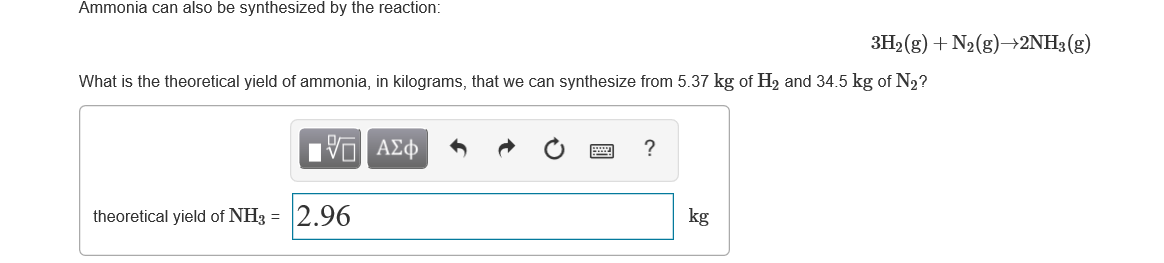
Transcribed Image Text:Ammonia can also be synthesized by the reaction:
3H2 (g) + N2(g)→2NH3(g)
What is the theoretical yield of ammonia, in kilograms, that we can synthesize from 5.37 kg of H, and 34.5 kg of N2?
Ηνα ΑΣφ
theoretical yield of NH3 =
2.96
kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning