
Concept explainers
The equation for the oxidation of phosphorus in air is P4(s) + 5 O2(g) P4O10(s). Identify the reactants and products and the stoichiometric coefficients. To what do the designations s and g refer?
Interpretation:
The reactant, product and stoichiometric coefficient should be identified. Designation for the g and s should be given.
Concept Introduction:
The law of conservation of mass states that no atoms can be created or destroyed in a chemical reaction, therefore, the number of atoms present in the reactants is equal to the number of atoms present in the products.
Answer to Problem 1PS
The reactant, product and stoichiometric coefficient are given below (a) Designation for the g and s is given below.
Reactant
Phosphorus and oxygen
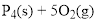
Product
Tetra phosphorus decaoxide
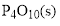
Stoichiometric coefficient are 1,5,1.

Designation for the g is gas state and s is solid state (b).
Explanation of Solution
The given equation is shown below using correct molecular formula,

In a given reaction left side of the reaction is called reactant and the right side of the reaction is called product. Therefore,
Reactant
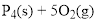
Phosphorus and oxygen
Product
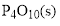
For balancing the reaction, stoichiometric coefficient of the reaction is needed therefore the stoichiometric coefficient of the reaction are 1,5,1 and is shown below,

Designation for the g is gas state is given below,
g and s state of the constituent. Therefore g indicates gas state and s indicates solid state.
The reactant, product and stoichiometric coefficient should be identified. Designation for the g and s were given.
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry & Chemical Reactivity
- A rebreathing gas mask contains potassium superoxide, KO2, which reacts with moisture in the breath to give oxygen. 4KO2(s)+2H2O(l)4KOH(s)+3O2(g) Estimate the grams of potassium superoxide required to supply a persons oxygen needs for one hour. Assume a person requires 1.00 102 kcal of energy for this time period. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 1.00 102 kcal of heat, calculate the amount of oxygen consumed and hence the amount of KO2 required. The ff0 for glucose(s) is 1273 kJ/mol.arrow_forwardWhen one mole of ethylene gas, C2H4, reacts with fluorine gas, hydrogen fluoride and carbon tetrafluoride gases are formed and 2496.7 kJ of heat are given off. What is Hf for CF4(g)?arrow_forwardA power plant is driven by the combustion of a complex fossil fuel having the formula C11H7S. Assume the air supply is composed of only N2 and O2 with a molar ratio of 3.76:1.00, and the N2 remains unreacted. In addition to the water produced, the fuels C is completely combusted to CO2 and its sulfur content is converted to SO2. In order to evaluate gases emitted at the exhaust stacks for environmental regulation purposes, the nitrogen supplied with the air must also be included in the balanced reactions. a Including the N2 supplied m the air, write a balanced combustion equation for the complex fuel assuming 100% stoichiometric combustion (i.e., when there is no excess oxygen in the products and the only C-containing product is CO2). Except in the case of N2, use only integer coefficients. b Including N2 supplied in the air, write a balanced combustion equation for the complex fuel assuming 120% stoichiometric combustion (i.e., when excess oxygen is present in the products and the only C-containing product is CO2). Except in the case of use only integer coefficients c Calculate the minimum mass (in kg) of air required to completely combust 1700 kg of C11H7S. d Calculate the air/fuel mass ratio, assuming 100% stoichiometric combustion. e Calculate the air/fuel mass ratio, assuming 120% stoichiometric combustion.arrow_forward
- Ethanol, C2H5OH, is a gasoline additive that can be produced by fermentation of glucose. C6H12O62C2H5OH+2CO2 (a) Calculate the mass (g) of ethanol produced by the fermentation of 1.000 lb glucose. (b) Gasohol is a mixture of 10.00 mL ethanol per 90.00 mL gasoline. Calculate the mass (in g) of glucose required to produce the ethanol in 1.00 gal gasohol. Density of ethanol = 0.785 g/mL. (c) By 2022, the U. S. Energy Independence and Security Act calls for annual production of 3.6 1010 gal of ethanol, no more than 40% of it produced by fermentation of corn. Fermentation of 1 ton (2.2 103 lb) of corn yields approximately 106 gal of ethanol. The average corn yield in the United States is about 2.1 105 lb per 1.0 105 m2. Calculate the acreage (in m2) required to raise corn solely for ethanol production in 2022 in the United States.arrow_forwardCarbon dioxide from the atmosphere weathers, or dissolves, limestone (CaCO3) by the reaction CaCO3(s)+CO2(g)+H2O(l)Ca2(aq)+2HCO3(aq) Obtain H for this reaction. See Table 6.2 for the data.arrow_forwardGraphite is burned in oxygen to give carbon monoxide and carbon dioxide. If the product mixture is 33% CO and 67% CO2 by mass, what is the heat from the combustion of 1.00 g of graphite?arrow_forward
- Chlorine dioxide, ClO2, is a reddish yellow gas used in bleaching paper pulp. The average speed of a ClO2 molecule at 25C is 306 m/s. What is the kinetic energy (in joules) of a ClO2 molecule moving at this speed?arrow_forwardInsoluble AgCl(s) precipitates when solutions of AgNO3(aq) and NaCl(aq) are mixed. AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq) rH = ? To measure the energy evolved in this reaction, 250. mL of 0.16 M AgNO3(aq) and 125 mL of 0.32 M NaCl(aq) are mixed in a coffee-cup calorimeter. The temperature of the mixture rises from 21.15 C to 22.90 C. Calculate the enthalpy change for the precipitation of AgCl(s), in kJ/mol. (Assume the density of the solution is 1.0 g/mL and its specific heat capacity is 4.2 J/g K.)arrow_forward4.19 How many metric tons of carbon are required to react with 7.83 metric tons of Fe2O3 according to the following reaction? 2Fe2O3+3C3CO2+4Fe How many metric tons of iron are produced?arrow_forward
- 4.69 The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce methanol, CH3OH. The box on the left represents the reactants at the instant of mixing, and the box on the right shows what is left once the reaction has gone to completion. Was there a limiting reactant in this reaction? If so, what was it? Write a balanced chemical equation for this reaction. As usual, your equation should use the smallest possible whole number coefficients for all substances.arrow_forwardEmissions from the burning of fossil fuels are often reported as gigatons ( Gt ) of carbon. In a calendar year, China emitted 2.9 Gt of carbon while the Unites States released 1.5 Gt of carbon. To what mass in gigatons of CO2CO2 do these amounts of carbon correspond?arrow_forwardThe sugar glucose (C6H12O6) is broken down in a decomposition reaction to ethanol (C2H6O) and bubbles, CO2. Write the balanced chemical equation for the reaction.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





