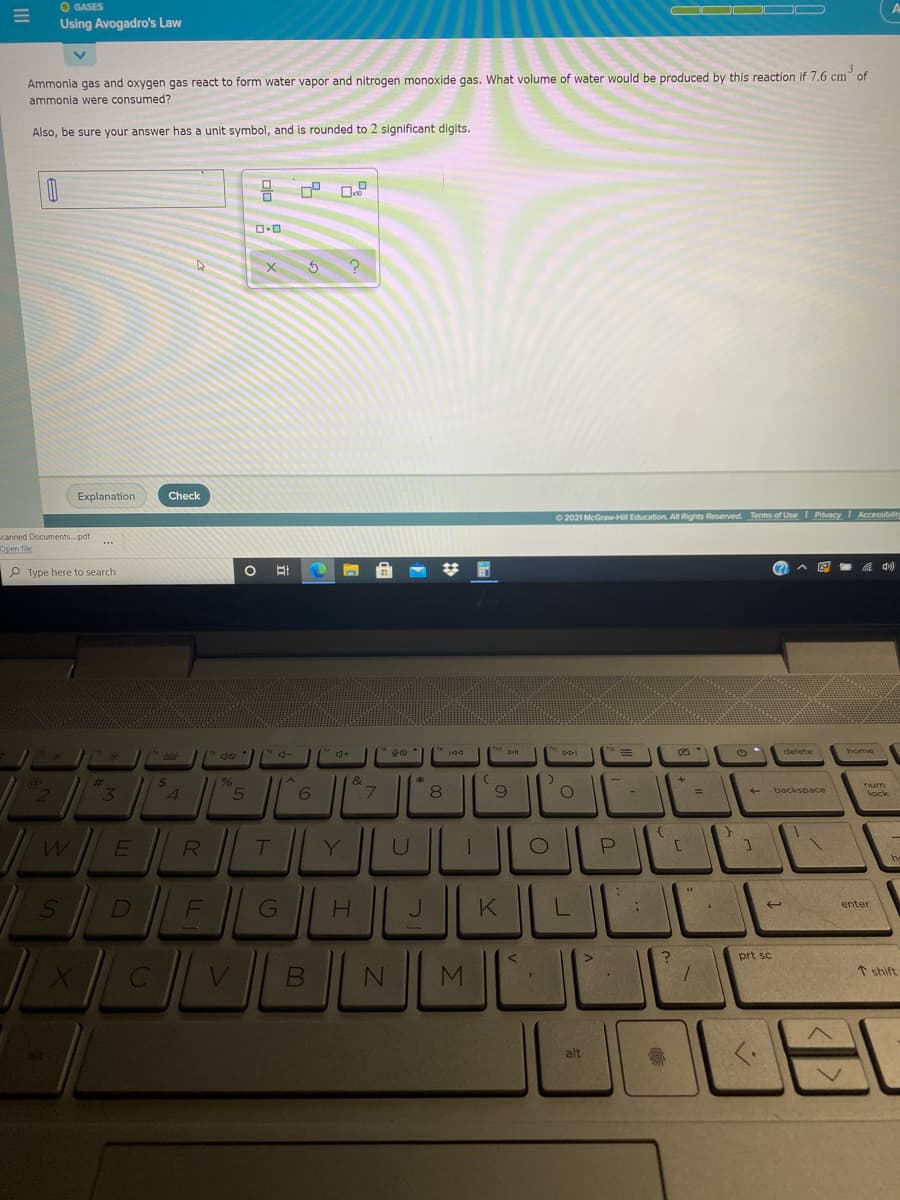
Ammonia gas and oxygen gas react to form water vapor and nitrogen monoxide gas. What volume of water would be produced by this reaction if 7.6 cm of ammonia were consumed?
Ammonia gas and oxygen gas react to form water vapor and nitrogen monoxide gas. What volume of water would be produced by this reaction if 7.6 cm of ammonia were consumed?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 74QAP: Magnesium ribbon reacts with acid to produce hydro- gen gas and magnesium ions. Different masses of...
Related questions
Question
Answer all questions please thank you ❤️❤️❤️
Transcribed Image Text:O GASES
Using Avogadro's Law
Ammonia gas and oxygen gas react to form water vapor and nitrogen monoxide gas. What volume of water would be produced by this reaction if 7.6 cm of
ammonia were consumed?
Also, be sure your answer has a unit symbol, and is rounded to 2 significant digits.
Explanation
Check
O 2021 McGrawH Education. All Rights Reserved. Terms of Use I Privacy Accessibility
canned Documents.pdf
Dpen fle
P Type here to search
* do
"DDI
delete
home
num
3.
4
8.
backspace
%3D
lock
w/ERTYUL
G
prt sc
* shift
alt
Transcribed Image Text:O GASES
Angel
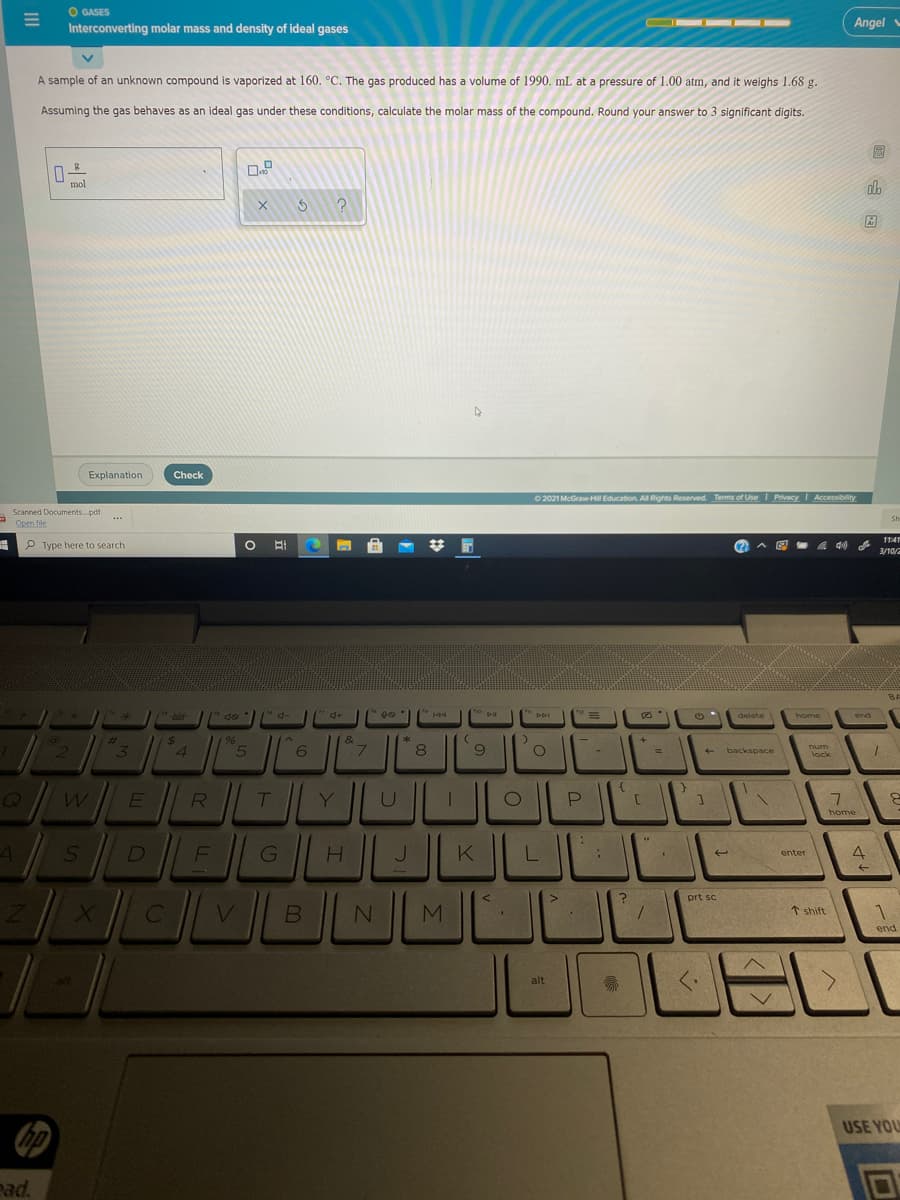
Interconverting molar mass and density of ideal gases
A sample of an unknown compound is vaporized at 160. °C. The gas produced has a volume of 1990. mL at a pressure of 1.00 atm, and it weighs 1.68 g.
Assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. Round your answer to 3 significant digits.
0-
mol
do
国
Explanation
Check
O 2021 McGrawHilI Education. All Rights Reserved. Terms of Use I Privacy I Accessibility
Scanned Documents. pdf..
Open file
Sh
# 國
11:4
P Type here to search
(?)
3/10/2
BA
no
DI
"DDI
home
199
delete
end
2$
4.
96
&
7
%23
+
5.
8
+
backspace
num
lock
R
Y
7.
home
DFGH
K
L
4
enter
prt sc
V.
1.
* shift
end
alt
の
USE YOU
ad.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
