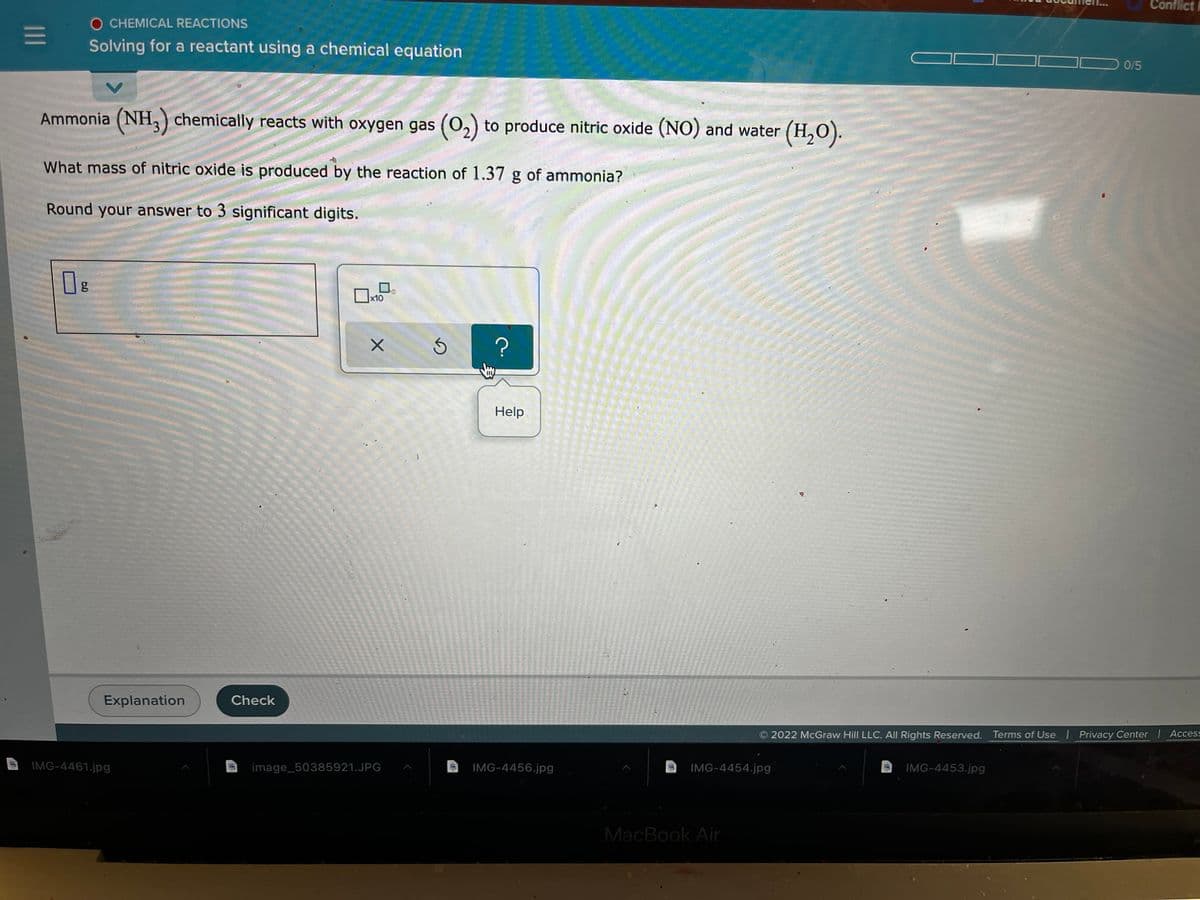
Ammonia (NH,) chemically reacts with oxygen gas (0,) to produce nitric oxide (NO) and water (H,O). What mass of nitric oxide is produced by the reaction of 1.37 g of ammonia? Round your answer to 3 significant digits.
Ammonia (NH,) chemically reacts with oxygen gas (0,) to produce nitric oxide (NO) and water (H,O). What mass of nitric oxide is produced by the reaction of 1.37 g of ammonia? Round your answer to 3 significant digits.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 67QAP: When potassium chlorate is subjected to high temperatures, it decomposes into potassium chloride and...
Related questions
Question
Transcribed Image Text:Conflict
O CHEMICAL REACTIONS
Solving for a reactant using a chemical equation
0/5
Ammonia (NH,) chemically reacts with oxygen gas (0,) to produce nitric oxide (NO) and water (H,O).
What mass of nitric oxide is produced by the reaction of 1.37 g of ammonia?
Round your answer to 3 significant digits.
x10
Help
Explanation
Check
2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Access
IMG-4461.jpg
image_50385921.JPG
IMG-4456.jpg
IMG-4454.jpg
IMG-4453.jpg
MacBook Air
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning