Using Hess's Law tó calculate net reaction enthalpy 1/5 The lead-acid storage battery is the oldest rechargeable battery in existence. It was invented in 1859 by French physician Gaston Plante and still retains application today, more than 150 years later. There are two reactions that take place during discharge of the lead-acid storage battery. In one step, sulfuric acid decomposes to form sulfur'trioxide and water: H,SO,(1) SO,(9) + H,O(1) AH=+113. kJ In another step, lead, lead(IV) oxide, and sulfur trioxide react to form lead(II) sulfate: Pb(s) + PbO,(s) + 2 SO,(g) 2 PbSO,(s) AH=-775. kJ Calculate the net change in enthalpy for the formation of one mole of lead(II) sulfate from lead, lead(IV) oxide, and sulfuric acid from these reactions. Round your answer to the nearest kJ. Explanation Check © 2022 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Center IMG-4423.jpg IMG-4422.jpg D IMG-4420.jpg Exam 2 review.doc IMG-4424.jpg
Using Hess's Law tó calculate net reaction enthalpy 1/5 The lead-acid storage battery is the oldest rechargeable battery in existence. It was invented in 1859 by French physician Gaston Plante and still retains application today, more than 150 years later. There are two reactions that take place during discharge of the lead-acid storage battery. In one step, sulfuric acid decomposes to form sulfur'trioxide and water: H,SO,(1) SO,(9) + H,O(1) AH=+113. kJ In another step, lead, lead(IV) oxide, and sulfur trioxide react to form lead(II) sulfate: Pb(s) + PbO,(s) + 2 SO,(g) 2 PbSO,(s) AH=-775. kJ Calculate the net change in enthalpy for the formation of one mole of lead(II) sulfate from lead, lead(IV) oxide, and sulfuric acid from these reactions. Round your answer to the nearest kJ. Explanation Check © 2022 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Center IMG-4423.jpg IMG-4422.jpg D IMG-4420.jpg Exam 2 review.doc IMG-4424.jpg
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.31QP: Chemical reactions are run in each of the beakers depicted below (labeled A, B, and C). The...
Related questions
Question
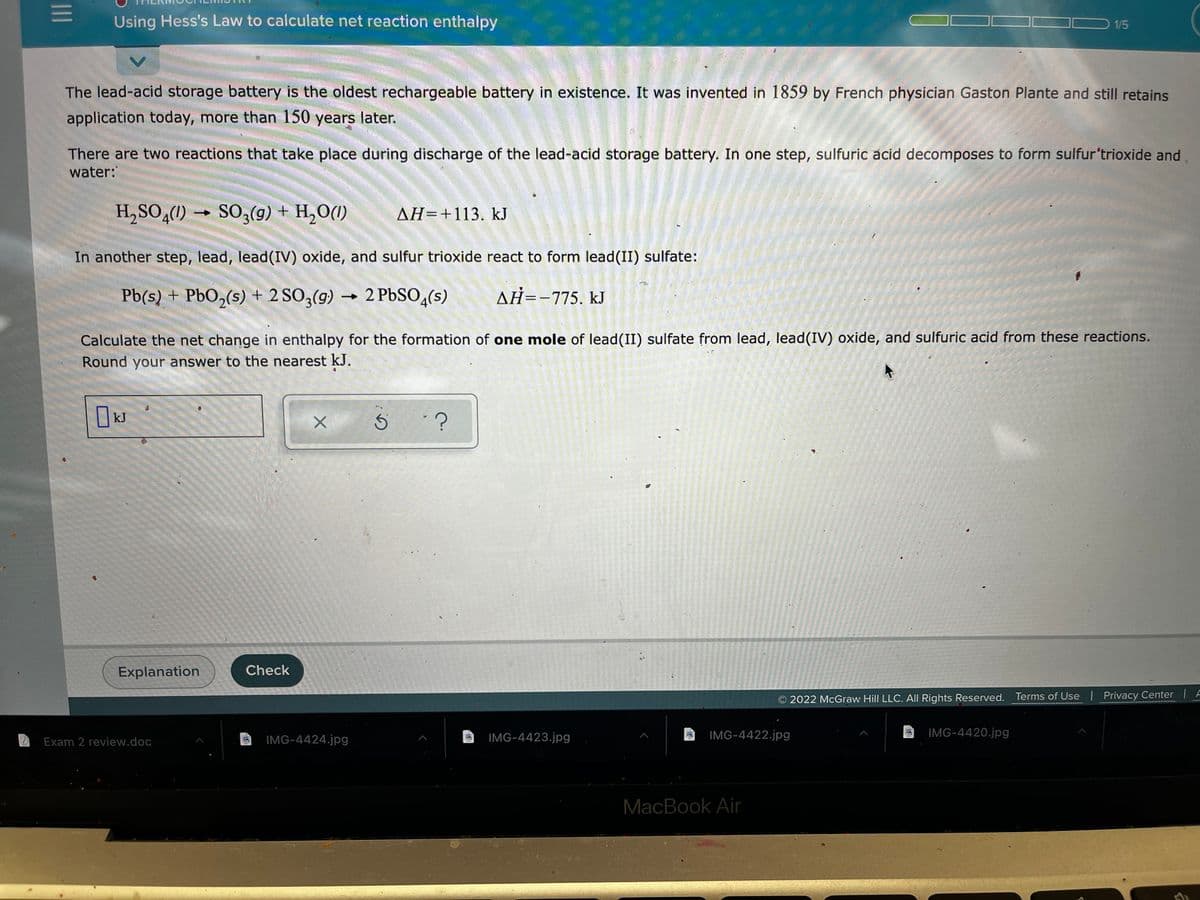
Transcribed Image Text:Using Hess's Law to calculate net reaction enthalpy
1/5
The lead-acid storage battery is the oldest rechargeable battery in existence. It was invented in 1859 by French physician Gaston Plante and still retains
application today, more than 150 years later.
There are two reactions that take place during discharge of the lead-acid storage battery. In one step, sulfuric acid decomposes to form sulfur'trioxide and
water:
LEGO
H,SO,(1) →
SO;(9) + H,O(1)
AH=+113. kJ
In another step, lead, lead(IV) oxide, and sulfur trioxide react to form lead(II) sulfate:
Pb(s) + PbO,(s) + 2 SO3(g)
2 PBSO,(s)
AH=-775, kJ
Calculate the net change in enthalpy for the formation of one mole of lead(II) sulfate from lead, lead(IV) oxide, and sulfuric acid from these reactions.
Round your answer to the nearest k.J.
kJ
Explanation
Check
2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | A
IMG-4424.jpg
IMG-4423.jpg
IMG-4422.jpg
IMG-4420.jpg
Exam 2 review.doc
MacBook Air
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning