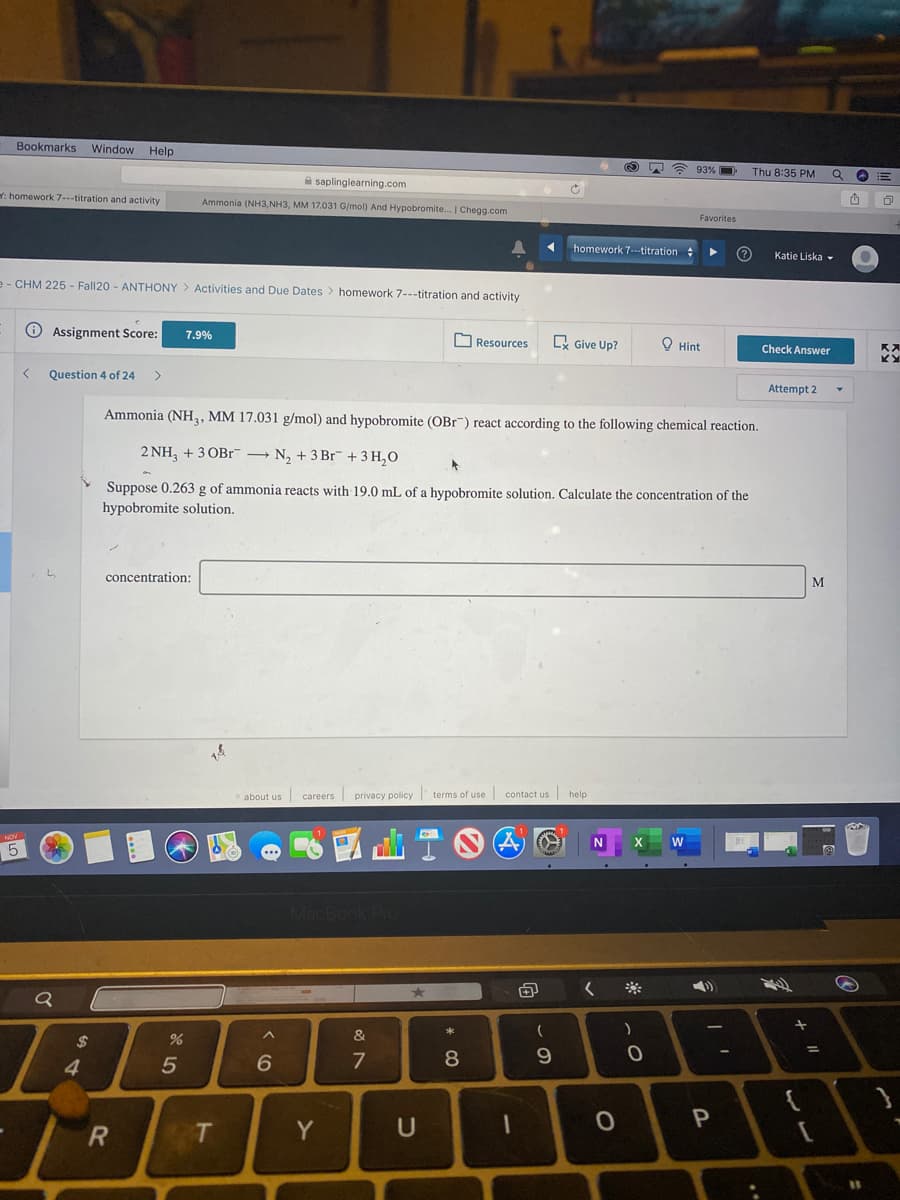
Ammonia (NH,, MM 17.031 g/mol) and hypobromite (OBr") react according to the following chemical reaction. 2 NH, + 3 OBr → N, + 3 Br + 3 H,O Suppose 0.263 g of ammonia reacts with 19.0 mL of a hypobromite solution. Calculate the concentration of the hypobromite solution. concentration: M
Ammonia (NH,, MM 17.031 g/mol) and hypobromite (OBr") react according to the following chemical reaction. 2 NH, + 3 OBr → N, + 3 Br + 3 H,O Suppose 0.263 g of ammonia reacts with 19.0 mL of a hypobromite solution. Calculate the concentration of the hypobromite solution. concentration: M
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter24: Coulometry
Section: Chapter Questions
Problem 24.12QAP
Related questions
Question
Ammonia (NH3, MM 17.031 g/mol) and hypobromite (OBr-) react according to the following chemical reaction .
2 NH3 + 3 OBr- —> N2 + 3Br- + 3 H2O
Suppose 0.263 g of ammonia react with 19.0 mL of hypobromite solution. Calculate the concentration of the hypobromite solution
Transcribed Image Text:Bookmarks
Window
Help
93% O
Thu 8:35 PM
A saplinglearning.com
: homework 7---titration and activity
Ammonia (NH3,NH3, MM 17.031 G/mol) And Hypobromite. | Chegg.com
Favorites
homework 7--titration
Katie Liska -
e - CHM 225 - Fall20 - ANTHONY > Activities and Due Dates > homework 7---titration and activity
O Assignment Score:
7.9%
Resources
R Give Up?
O Hint
Check Answer
Question 4 of 24
>
Attempt 2
Ammonia (NH, MM 17.031 g/mol) and hypobromite (OBr") react according to the following chemical reaction.
2 NH, + 3 OBr¯ → N, + 3 Br + 3 H,O
Suppose 0.263 g of ammonia reacts with 19.0 mL of a hypobromite solution. Calculate the concentration of the
hypobromite solution.
concentration:
M
about us careers privacy policy terms of use contact us help
5
&
%24
4
6.
7
8
9.
Y
U
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
