Among the "Greenhouse Gases" carbon dioxide is the one that contributes the most. If 110 g of this gas confined in a 5.00 L container at 177"C then compare its pressure with that predicted by the ideal gas and find out if this gas behaving ideally? (Some other informations are mention in the following table. van der Waals Constants of Some Common Gases a. atm · L2 mol Gas mol He 0.034 0.0237 Ne 0.211 0.0171 Ar 1.34 0.0322 Kr 2.32 0.0398 Xe 4.19 0.0266 0.0266 H2 N2 0.244 1.39 0.0391 0.0318 02 Cl, CO2 CH, 1.36 6.49 0.0562 3.59 0.0427 2.25 0.0428 CCL 20.4 0.138 4.17 0.0371 NH, HO 5.46 0.0305 Select one: O a. To some extent it behave ideally O b. No, it does not behave ideally O c. Yes, it does behave ideally O d. Not enough information to solve this question O e. Being greenhouse Gases, it behave ideally in all conditions
Among the "Greenhouse Gases" carbon dioxide is the one that contributes the most. If 110 g of this gas confined in a 5.00 L container at 177"C then compare its pressure with that predicted by the ideal gas and find out if this gas behaving ideally? (Some other informations are mention in the following table. van der Waals Constants of Some Common Gases a. atm · L2 mol Gas mol He 0.034 0.0237 Ne 0.211 0.0171 Ar 1.34 0.0322 Kr 2.32 0.0398 Xe 4.19 0.0266 0.0266 H2 N2 0.244 1.39 0.0391 0.0318 02 Cl, CO2 CH, 1.36 6.49 0.0562 3.59 0.0427 2.25 0.0428 CCL 20.4 0.138 4.17 0.0371 NH, HO 5.46 0.0305 Select one: O a. To some extent it behave ideally O b. No, it does not behave ideally O c. Yes, it does behave ideally O d. Not enough information to solve this question O e. Being greenhouse Gases, it behave ideally in all conditions
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.21E: Pressures of gases in mixtures are referred to as partial pressures and are additive. 1.00 L of He...
Related questions
Question
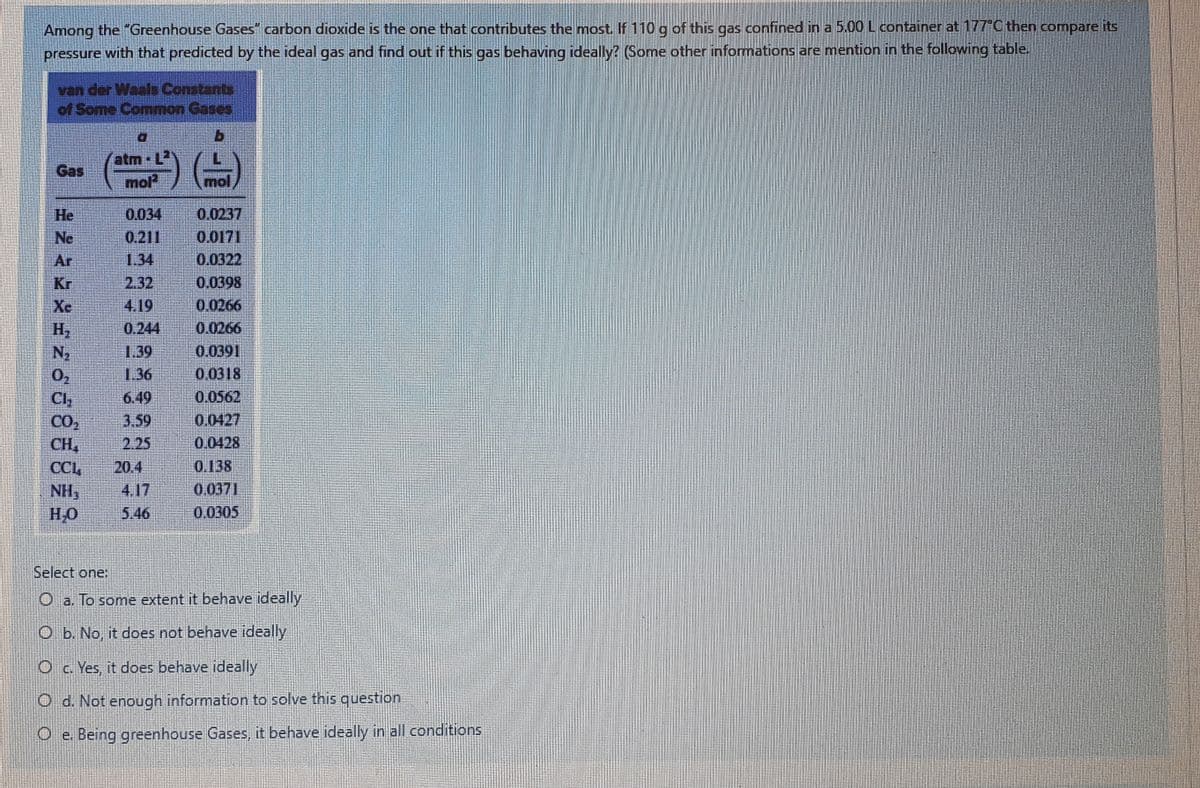
Transcribed Image Text:Among the "Greenhouse Gases" carbon dioxide is the one that contributes the most. If 110 g of this gas confined in a 5.00 L container at 177°C then compare its
pressure with that predicted by the ideal gas and find out if this gas behaving ideally? (Some other informations are mention in the following table.
van der Waals Constants
of Some Common Gases
atm L2
mol
Gas
0.034
0.211
1.34
2.32
0.0237
0.0171
0.0322
0.0398
0.0266
0.0266
0.0391
0.0318
0.0562
0.0427
Не
Ne
Ar
Kr
Xe
Hz
N2
4.19
0.244
1.39
1.36
Cl,
CO2
CH,
CCL,
NH3
6.49
3.59
2.25
0.0428
0.138
0.0371
20.4
4.17
но
5.46
0.0305
Select one:
O a. To some extent it behave ideally
O b. No, it does not behave ideally
O c. Yes, it does behave ideally
O d. Not enough information to solve this question
O e. Being greenhouse Gases, it behave ideally in all conditions
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax