Q: 2 NaCl(aq)+Ba(OH), (aq) BACL, + 2 N2OH Identify the precipitate, or lack thereof, for the reaction.…
A:
Q: A sample of sodium hydrogen carbonate solid weighing 0.1491 g requires 37.83 mL of a hydrochloric…
A: Given data contains, Mass of sodium hydrogen carbonate is 0.1491 g. Volume of hydrochloric acid is…
Q: Upon heating, 48.6 mL of water was evaporated from 180. mL of 0.302 M C6H12O6(aq). What is the…
A: Given that,concentration of C6H12O6 = 0.302 M = 0.302 mol/Lvolume of the solution = 180 mL = 0.18…
Q: Question attached
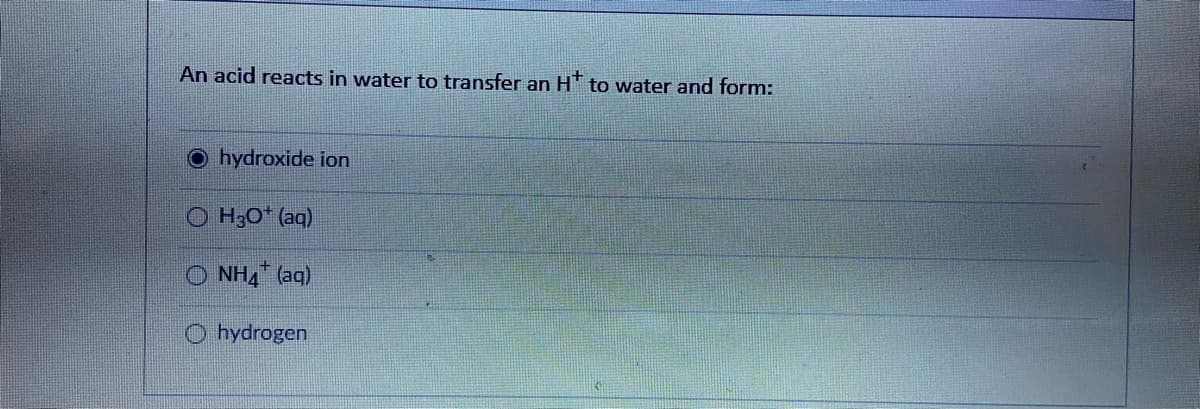
A: Volume of H3PO4 = 10 mL or 0.01 L Molarity of H3PO4 = 0.196 M Moles of H3PO4 =Molarity ×Volume=0.196…
Q: A 0.3146 g sample of a mixture of NaCl(s) and KBr(s) was dissolved in water. The resulting solution…
A: The reaction involves the determination of the unknown ions in a solution as their insoluble salt…
Q: A student wishes to determine the mass percentage of barium in a 2.016g sample. The student…
A: Given : Molarity of H2SO4 = 0.20M Volume of H2SO4 = 20mL
Q: One way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is…
A: Given: The mass of silver chloride (AgCl) is 8.5 mg The molarity of added silver nitrate solution is…
Q: The mass percent of acetic acid (CH3COOH) in a bottle of vinegar is 5.45% in water. What is the…
A: Consider the volume of the solution as 1 L (1000 mL).Determine the mass of l L of solution from the…
Q: A student wishes to determine the mass percentage of barium in a 2.016g sample. The student…
A: Given : Mass of sample = 2.016g Molarity of H2SO4 = 0.20M Volume of H2SO4 = 20ml
Q: Sodium carbonate, Na2CO3, can be titrated with HCl according to the following balanced chemical…
A: First, the given grams of sodium carbonate is converted into moles.
Q: What volume (in mL) of 0.197 mol/L sodium hydroxide, NaOH (aq), is needed to completely neutralize…
A: Given: Concentration of NaOH = 0.197 M Concentration of HCl = 0.298 M And volume of HCl solution to…
Q: A 20.05 mL sample of Vinegar(an aqueous solution of acetic acid, HC2H3O2) has a density of…
A: Find mas of vinegar present in 20.05 ml sample of vinegar Density of sample is 1.061 g/mLmass of…
Q: Lactic acid (HC3H5O3) is a monoprotic acid that forms when milk becomes sour. A 40.00 mL sample of…
A:
Q: How many moles of potassium hydrogen phosphate (K2HPO4) are there if some potassium hydrogen…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: the following acid-base neutralization, 1.68 g of the solid acid HC6H5O (94.12 g/mol) neutralized…
A: Moles of acid = mass / molar mass = 1.68 / 94.12 = 0.01785 approx Since from the reaction, 1 mole of…
Q: 4. What volume of 0.1000 mol/L. NaOH(aq) is needed to react completely with 20 mL of 0.1805 mol/L…
A:
Q: To determine the amount of iron in a dietary supplement, a random sample of 15 tablets weighing a…
A: Given data: Total mass of the sample = 20.505 g Mass of sample dissolved in Fe(OH)3 = 3.116 g Mass…
Q: he endpoint with 190. ml of 0.4000 Mhydrogen chloride (HCI) solution. balanced chemical equation for…
A: Given reaction is an acid-base reaction in which HCL acid and carbonate is a base because acid gives…
Q: A key step in balancing chemical equations is correctly identifyingthe formulas of the reactants and…
A: (a) The balanced chemical equation for this combination reaction, having correctly identified the…
Q: Consider these compounds: A. Cas B. Ag,Cro4 C. Fes D. Zn(OH)2 Complete the following statements by…
A: Solubility of compounds can be compared on the basis of their Ksp ( solubility product constant )…
Q: (ii) A student was given 400 cm of aqueous ammonia solution, NH, (aq). The student was asked to…
A: The balanced reaction given is: 2NH3(aq)+H2SO4(aq)→(NH4)2SO4 Conversion unit: 1 L=1 dm31 L=1000 cm3…
Q: 3. With 85 % H2SO, by volume in water being used in the reaction, how many moles of water will be…
A: Since you have asked multiple questions, we will solve one question for you. If you want any…
Q: A particular solution of NaOH is supposed to be approximately 0.100 M. To determine the exact…
A:
Q: 12.) A 2.00g sample of limestone was dissolved in hydrochloric acid and all the calcium present in…
A: Mass of sample of limestone = 2.00 g Mass of precipited , CaC2O4 = 2.43 g Ammonium oxalate is…
Q: A mixture of barium chloride and sulfuric acid yield the precipitate barium sulfate and hydrochloric…
A: Given: Sulfuric Acid is limiting reagent, 39.5 grams of barium sulfate are produced. Molar mass of…
Q: Excess Na2SO4(aq) is added to a 2.43×102 mL sample of industrial waste containing Ba2+ ions. If 20.5…
A: Total industrial sample = 2.43 x 102 mLTotal mass of BaSO4 precipitated= 20.5gThe given reaction is
Q: In the analysis of an iron ore by precipitation of the iron as Fe(OH)3, what weight of the sample…
A: Mass% is equal to ratio of the mass of the substance to the total mass of the substance multiplied…
Q: Determine the mass in grams of HCI that can react with 0.750 g of Al(OH), açcording to the following…
A:
Q: Balance the following equation and charges, Na + H2O--> Na^+ + H2 + OH^-
A: Given: Reaction of sodium with water To find: Balance it and balance the charges as well Solution:…
Q: Complete the followin reaction by providing the products formed. Then balance the reaction and write…
A: The above given reaction is a double displacement reaction. Hence the products forming will be…
Q: Consider the reaction. 2 NaCl(aq)+Ba(OH),(aq) BaCl, + 2 NAOH Identify the precipitate, or lack…
A:
Q: In the "Methodé Champenoise," grape juice is fermented in a wine bottle to produce sparkling wine.…
A:
Q: The town of Andover obtains drinking water from three sources that together produce up to 6.81 × 107…
A:
Q: You have an aqueous solution containing one or more of the following anions CO32*, CI', r and SO42.…
A: Here, the ions present are: CO32-, Cl-, I- and SO42-. To the above ions, Ba(NO3)2 is added, a…
Q: A fertilizer railroad car carrying 34,300 gallons of commercialaqueous ammonia (30% ammonia by…
A: In order to calculate the amount of citric acid required to neutralize the spill we first write down…
Q: The Ca in a 200.0-mL sample of natural water was determined by precipitating the cation as CaC,O4…
A: Mass of empty crucible ( A )= 26.6002 g Mass of crucible + CaO ( B) = 26.7134 g Mass of CaO = B…
Q: What volume. (in mL) of 0.400 mol/L sodium hydroxide, NAOH (aq), is needed to completely neutralize…
A: Given :- Molarity of NaOH solution = 0.400 mol/L Molarity of HCl solution = 0.261 mol/L Volume of…
Q: A 0.0200 M aqueous solution of sodium sulfate solution was prepared in a 50.00-mL volumetric flask.…
A: On dilution number of moles remain same so we can write M1V1= M2V2 M1 ,V1 are molarity and volume…
Q: Consider the reaction CuO(s) + H2(g) → Cu(s) + H2O(1) In this reaction, which substances are the…
A: oxidation → Increase in oxidation Number .Reduction → Decrease in oxidation Number.Oxidisingagent →…
Q: The substance methylamine is a weak base like ammonia. Write an equation for the reaction that takes…
A:
Q: 2. 19.32 mL of 0.500 M NaOH solution is required to exactly neutralize a 10.00 mL sample of vinegar.…
A: As per our guidelines, we are supposed to answer only one question. Kindly repost other question as…
Q: What weight (in grams) of oxalic acid (MW= 90 g/mol) will be required to prepare 1 liter of 0.25 N?
A:
Q: An aqueous solution of ammonium sulfate is allowed to react with an aqueous solution of lead(II)…
A:
Q: In the reaction, KHS(aq) + HCI(aq) → KCI(aq) + H2S(g), which ions are the spectator ions O HS and CI…
A:
Q: The mass percent of concentrated H 2SO 4 (MW=98.08 g/mol) is 96.0% and its density is 1.84 g/mL.…
A:
Q: The balanced equation for the reaction of aqueous Pb(ClO,), with aqueous NaI is Pb(CIO,),(aq) + 2…
A: Given: Pb(ClO3)2 (aq) + 2 NaI (aq) ------> PbI2 (s) + 2 NaClO3 (aq) Volume of Pb(ClO3)2 = 1.50 L…
Q: A reaction is performed where 10.0 g K2S(s) are added to 250.0 mL of a 0.855 M HCl (aq) solution.…
A: The balance chemical reaction , K2S + 2HCl = H2S + 2KCl…
Q: When concentrated CaCl2 (aq) is added to NazHPO, (aq), a white precipitate forms that is 38.7% Ca by…
A: 1. During a chemical reaction, if two ions, having different reactivity exchanges there position…
Q: Vinegar contains acetic acid (HC2H3O2), which is responsible for its acidity. In one analysis of a…
A:
Q: The amount of nitrogen in an organic substance can be determined by an analytical method called the…
A:
Step by step
Solved in 2 steps

- Gold metal will dissolve only in aqua regia, a mixture of concentrated hydrochloric acid and concentrated nitric acid in a 3:1 volume ratio. The products of the reaction between gold and the concentrated acids are AuCl4-(aq), NO(g), and H2O. The equation for this reaction where HNO3 and HCl are strong acids is Au(s)+4Cl(aq)+4H+(aq)+NO3(aq)AuCl4(aq)+NO(g)+2H2O(a) What stoichiometric ratio of hydrochloric acid to nitric acid should be used? (b) What volumes of 12 M HCl and 16 M are HNO3 required to furnish the Cl- and NO3- ions to react with 25.0 g of gold?1. Sometimes a reaction can fall in more than one category. Into what category (or categories) does the reaction of Ba(OH)2(aq) + H+PO4(aq) fit? acid-base and oxidation-reduction oxidation-reduction acid-base and precipitation precipitationOn Easter Sunday, April 3, 1983, nitric acid spilled from a tank car near downtown Denver, Colorado. The spill was neutralized with sodium carbonate: 2HNO3(aq)+Na2CO3(aq)2NaNO3(aq)+H2O(l)+CO2(g) a. Calculate H for this reaction. Approximately 2.0 104 gal nitric acid was spilled. Assume that the acid was an aqueous solution containing 70.0% HNO3 by mass with a density of 1.42 glcm3. What mass of sodium carbonate was required for complete neutralization of the spill, and what quantity of heat was evolved? (Hf for NaNO3(aq) = 467 kJ/mol) b. According to The Denver Post for April 4, 1983, authorities feared that dangerous air pollution might occur during the neutralization. Considering the magnitude of H, what was their major concern?
- Ten mL of concentrated H3PO4 (91.7% by mass, d=1.69g/mL) was accidentally poured into a beaker containing 20.0 g of NaOH. Not all the NaOH was consumed. How many grams of NaOH were left unreacted? The equation for the reaction is H3PO4(aq)+3OH(aq)3H2O+PO43(aq)Many over-the-counter antacid tablets are now formulated using calcium carbonate as die active ingredient, which enables such tablets to also be used as dietary calcium supplements. As an antacid for gastric hyperacidity, calcium carbonate reacts by combining with hydrochloric acid found in the stomach, producing a solution of calcium chloride, converting die stomach acid to water, and releasing carbon dioxide gas (which the person suffering from stomach problems may feel as a burp). Write die balanced chemical equation for this process.3.14 A number of compounds are used in cement, and reactions among them occur when water is added. In one, CaO reacts with Al2O3 and water to form Ca3Al2(OH)12. Write a bal- anced chemical equation for this process.
- Chlorine gas was first prepared in 1774 by C. W. Scheele by oxidizing sodium chloride with manganese(IV) oxide. The reaction is NaCl(aq)+H2SO4(aq)+MnO2(s)Na2SO4(aq)+MnCl2(aq)+H2O(l)+Cl2(g) Balance this equation.What volume of 0.600 M HCl is required to react completely with 2.50 g of sodium hydrogen carbonate? NaHCO3(aq)+HCl(aq)NaCl2(g)+CO2(l)Triiodide ions are generated in solution by the following (unbalanced) reaction in acidic solution: IO3(aq) + I(aq) I3(aq) Triiodide ion concentration is determined by titration with a sodium thiosulfate (Na2S2O3) solution. The products are iodide ion and tetrathionate ion (S4O6). a. Balance the equation for the reaction of IO3 with I ions. b. A sample of 0.6013 g of potassium iodate was dissolved in water. Hydrochloric acid and solid potassium iodide were then added. What is the minimum mass of solid KI and the minimum volume of 3.00 M HQ required to convert all of the IO3 ions to I ions? c. Write and balance the equation for the reaction of S2O32 with I3 in acidic solution. d. A 25.00-mL sample of a 0.0100 M solution of KIO. is reacted with an excess of KI. It requires 32.04 mL of Na2S2O3 solution to titrate the I3 ions present. What is the molarity of the Na2S2O3 solution? e. How would you prepare 500.0 mL of the KIO3 solution in part d using solid KIO3?
- 4-81 (Chemical Connections 4C) Balance the lithium iodine battery redox reaction described in this sec tion and identify the oxidizing and reducing agents present.4.112 A metallurgical firm wishes to dispose of 1300 gallons of waste sulfuric acid whose molarity is 1.37 M. Before disposal, it will be reacted with calcium hydroxide (slaked lime), which costs $0.23 per pound. (a) Write the balanced chemical equation for this process. (b) Determine the cost that the firm will incur from this use of slaked lime.What volume of 0.0521 M Ba(OH)2 is required to neutralize exactly 14.20 mL of 0.141 M H3PO4? Phosphoric acid contains three acidic hydrogens.











