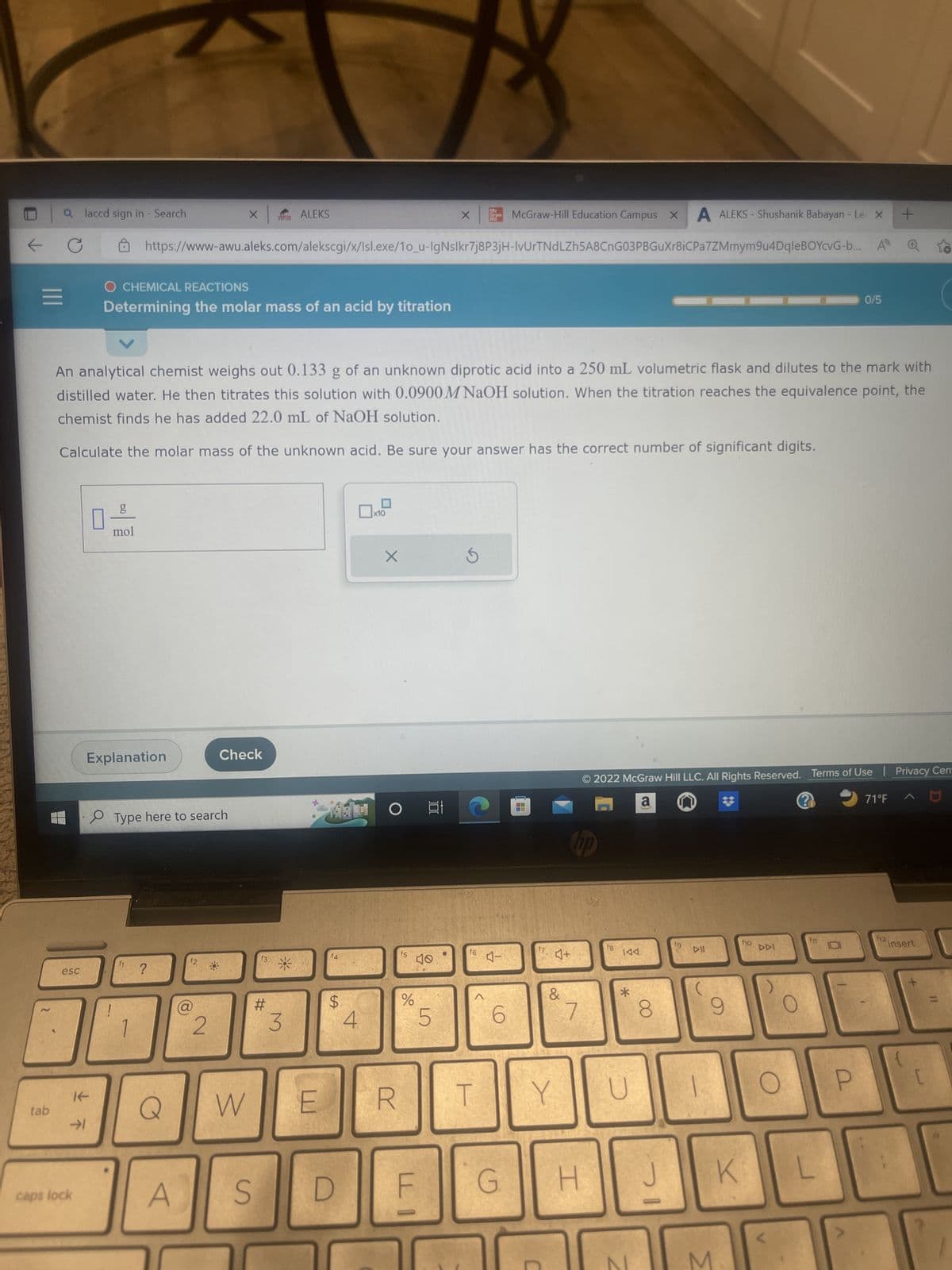
An analytical chemist weighs out 0.133 g of an unknown diprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. He then titrates this solution with 0.0900 M NaOH solution. When the titration reaches the equivalence point, the chemist finds he has added 22.0 mL of NaOH solution. Calculate the molar mass of the unknown acid. Be sure your answer has the correct number of significant digits. g mol 0 X
An analytical chemist weighs out 0.133 g of an unknown diprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. He then titrates this solution with 0.0900 M NaOH solution. When the titration reaches the equivalence point, the chemist finds he has added 22.0 mL of NaOH solution. Calculate the molar mass of the unknown acid. Be sure your answer has the correct number of significant digits. g mol 0 X
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 118CP: Malonic acid (HO2CCH2CO2H) is a diprotic acid. In the titration of malonic acid w ith NaOH,...
Related questions
Question
Make sure correct significant answer must be
Transcribed Image Text:□a laccd sign in - Search
←
|||
tab
esc
K
caps lock
F
0
V
O CHEMICAL REACTIONS
Determining the molar mass of an acid by titration
g
mol
An analytical chemist weighs out 0.133 g of an unknown diprotic acid into a 250 mL volumetric flask and dilutes to the mark with
distilled water. He then titrates this solution with 0.0900 M NaOH solution. When the titration reaches the equivalence point, the
chemist finds he has added 22.0 mL of NaOH solution.
Calculate the molar mass of the unknown acid. Be sure your answer has the correct number of significant digits.
!
1
-
Explanation
https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym9u4DqleBOYcVG-b...
?
Type here to search
Q
A
x
2
2
Check
W
S
ALEKS
f3
#
3
E
f4
LA
$
D
4
X
R
15
40
%
Et
LLI
Mc
X Graw
5
16
T
AI
McGraw-Hill Education Campus X A ALEKS- Shushanik Babayan - Le X +
4
6
G
1881
4+
C
&
Y
no
7
H
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Cen
a
^ U
?
18
IAA
*
U
8
J
6
1
9
3
K
f10
O
0/5
L
P
71°F
12
insert
[
11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning