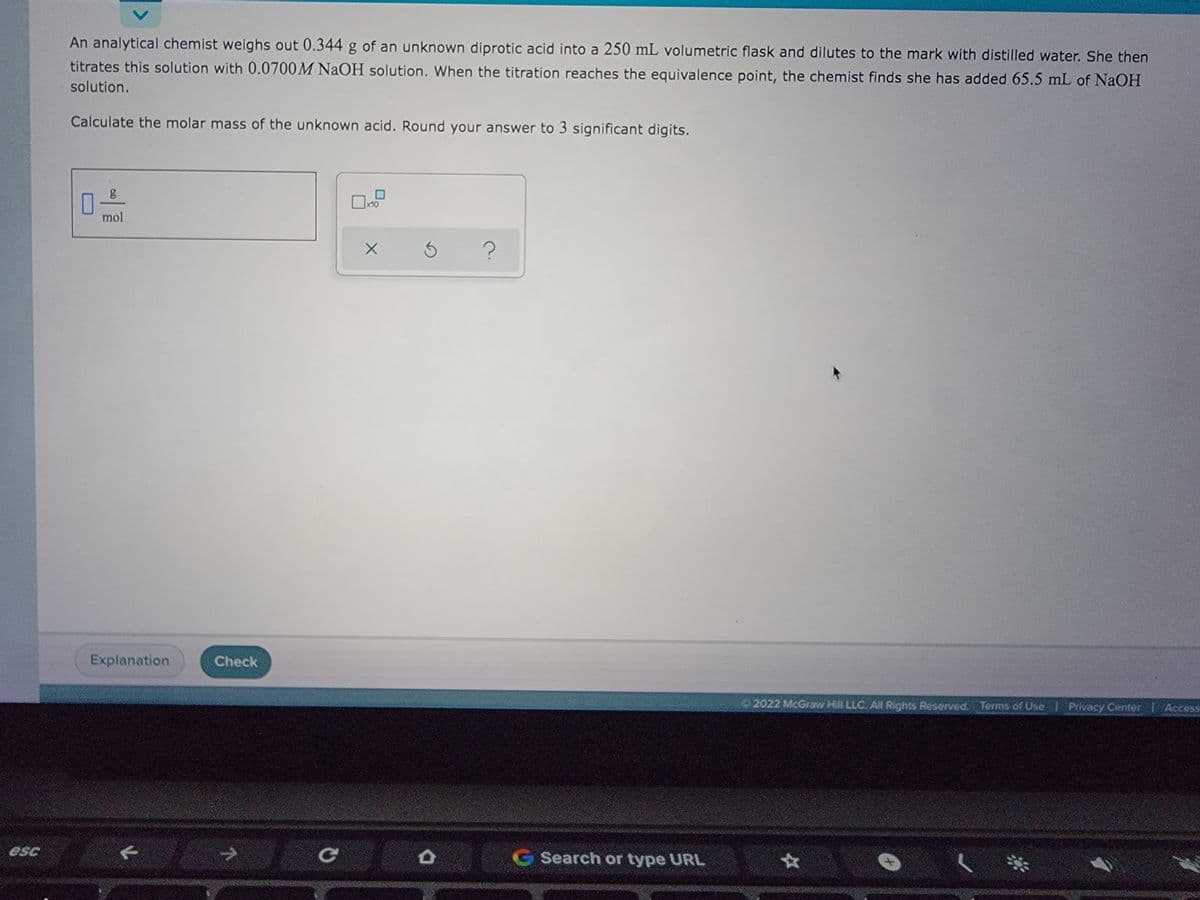
An analytical chemist weighs out 0.344 g of an unknown diprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. She then titrates this solution with 0.0700M NaOH solution. When the titration reaches the equivalence point, the chemist finds she has added 65.5 mL of NaOH solution. Calculate the molar mass of the unknown acid. Round your answer to 3 significant digits. mol X 3 ?
An analytical chemist weighs out 0.344 g of an unknown diprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. She then titrates this solution with 0.0700M NaOH solution. When the titration reaches the equivalence point, the chemist finds she has added 65.5 mL of NaOH solution. Calculate the molar mass of the unknown acid. Round your answer to 3 significant digits. mol X 3 ?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 97AE: A student intends to titrate a solution of a weak monoprotic acid with a sodium hydroxide solution...
Related questions
Question
Transcribed Image Text:esc
V
An analytical chemist weighs out 0.344 g of an unknown diprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. She then
titrates this solution with 0.0700M NaOH solution. When the titration reaches the equivalence point, the chemist finds she has added 65.5 mL of NaOH
solution.
Calculate the molar mass of the unknown acid. Round your answer to 3 significant digits.
g
0x10
mol
X
3
?
Explanation
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Access
Check
C
D
C Search or type URL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning