An arctic weather balloon is filled with 17.6 L of helium gas inside a prep shed. The temperature inside the shed is 6. °C. The balloon is then taken outside, where the temperature is -24. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Round your answer to 3 significant digits. x10 olo Ar
An arctic weather balloon is filled with 17.6 L of helium gas inside a prep shed. The temperature inside the shed is 6. °C. The balloon is then taken outside, where the temperature is -24. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Round your answer to 3 significant digits. x10 olo Ar
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section8.4: Gas Density, Molar Mass, And The Ideal Gas Law
Problem 8.9CE
Related questions
Question
My final answer was 15.7, I just want to check if it's correct.
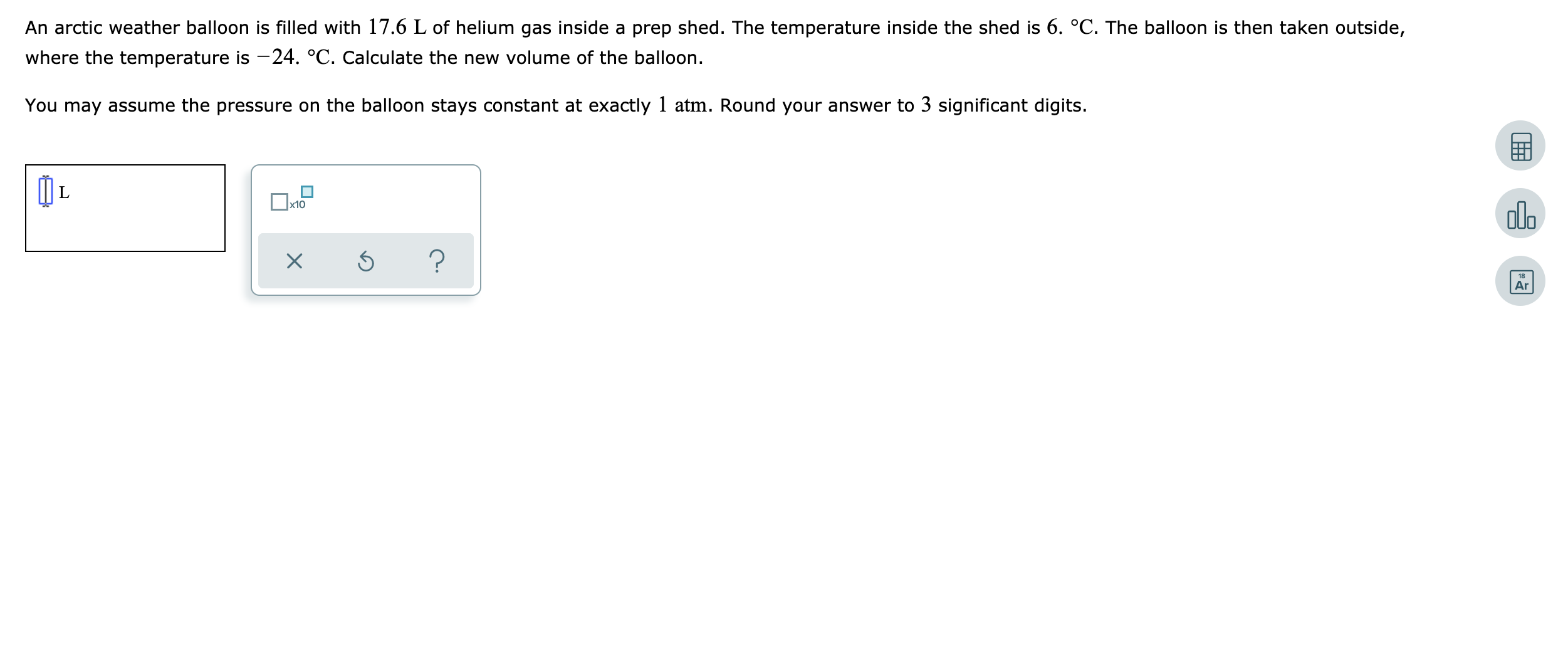
Transcribed Image Text:An arctic weather balloon is filled with 17.6 L of helium gas inside a prep shed. The temperature inside the shed is 6. °C. The balloon is then taken outside,
where the temperature is -24. °C. Calculate the new volume of the balloon.
You may assume the pressure on the balloon stays constant at exactly 1 atm. Round your answer to 3 significant digits.
x10
olo
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,