An electrochemical cell consists of a left compartment with a zinc electrode ih contact WILH compartment with a silver electrode in contact with 1.0 mol/L AgNO3(aq). The standard reduction potentials are: E° = 0.80 V Ag* +e = Ag Zn2* + 2e = Zn E° =-0.76 V When this cell is allowed to operate at 25°C, which of the following statements is true? The silver electrode will be the anode. B The concentration of Ag+ ions in right compartment will increase. C) The standard cell potential for this cell is 0.04 V. D The silver electrode will be the cathode. E zn* ions will be reduced to Zn metal.
An electrochemical cell consists of a left compartment with a zinc electrode ih contact WILH compartment with a silver electrode in contact with 1.0 mol/L AgNO3(aq). The standard reduction potentials are: E° = 0.80 V Ag* +e = Ag Zn2* + 2e = Zn E° =-0.76 V When this cell is allowed to operate at 25°C, which of the following statements is true? The silver electrode will be the anode. B The concentration of Ag+ ions in right compartment will increase. C) The standard cell potential for this cell is 0.04 V. D The silver electrode will be the cathode. E zn* ions will be reduced to Zn metal.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 159MP: A galvanic cell is based on the following half-reactions: In this cell, the copper compartment...
Related questions
Question
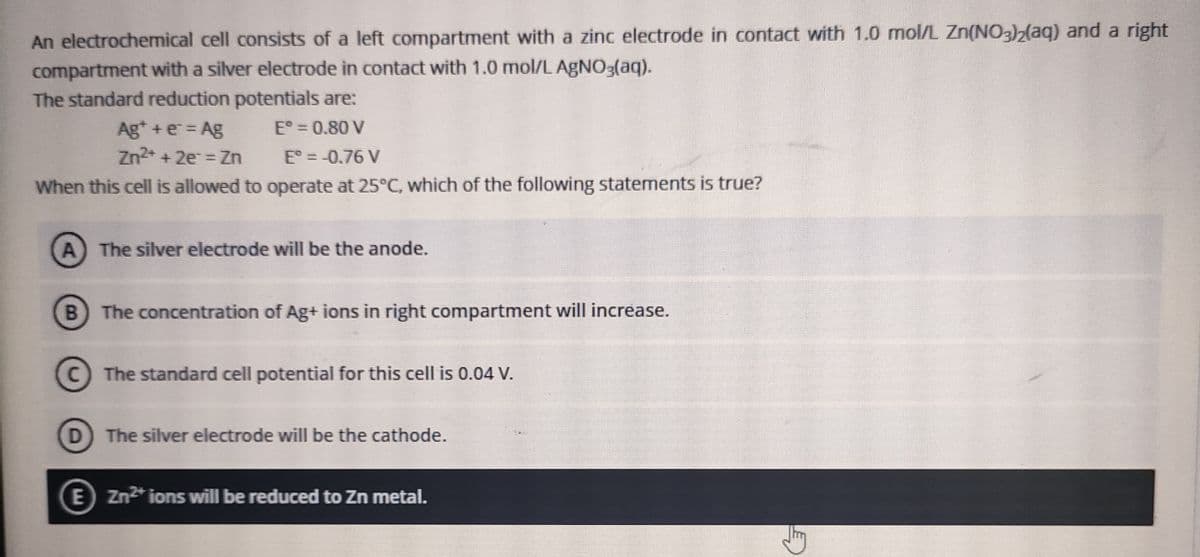
Transcribed Image Text:An electrochemical cell consists of a left compartment with a zinc electrode in contact with 1.0 mnol/L Zn(NO3)2(aq) and a right
compartment with a silver electrode in contact with 1.0 mol/L AGNO3(aq).
The standard reduction potentials are:
E° = 0.80 V
Ag* + e = Ag
Zn2* + 2e = Zn
E° = -0.76 V
When this cell is allowed to operate at 25°C, which of the following statements is true?
The silver electrode will be the anode.
B The concentration of Ag+ ions in right compartment will increase.
C The standard cell potential for this cell is 0.04 V.
D
The silver electrode will be the cathode.
E Zn2* ions will be reduced to Zn metal.

Transcribed Image Text:This error usually occurs only occasionally, is often large, and may cause a result to be either high or low, and this error leads to
outliers.
A) systematic error
absolute error
(C) gross error
D) indeterminate error
E) random error
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning