An elementary process has an activation energy of 88 kJ/mol. If the enthalpy change for the reaction is -60 kJ/mol, what is the activation energy for the reverse reaction? 148 kJ/mol -88 kJ/mol 60 kJ/mol -60 kJ/mol 28 kJ/mol
Q: Assign IUPAC names to the following amides: || CH;CH,CH-C-NH, a. CH,CH; b. CH;CH,CH,-C-NH- C-NH-CHCH...
A:
Q: Consider the following system at equilibrium where Kc = 1.80×10-4 and AH° = 92.7 kJ/mol at 298 K. NH...
A: Answer: Given reaction is shown below: NH4HS(s)↔NH3(g)+H2S(g) 1 mole of NH4HS is decomposing to give...
Q: Carbon dioxide gas is compressed at steady state from a pressure of 16 lb/in2 and a temperature of 3...
A: Introduction : Ideal gas equation is PV=nRT where P is pressure, V is volume and T is temperature o...
Q: Show complete solutions in correct problem solving format, using the correct units and number of sig...
A:
Q: Evaluate the chemical reactions carefully. Predict the reagents required (marked with letters) in or...
A: Given : structure of reactant and product.
Q: Part 1. TRUE or FALSE.Choose A if the statement is true. Choose D if the statement is false. 1. Base...
A: 1) D statement is false Lewis acid as an electrophile (which is electron poor), and a Lewis base as ...
Q: 9.) Predict the major product of the following reaction? H,50. Het
A:
Q: A total of 1.879 F of electricity (1 F = 1 mol e) was required to electrodeposit all of the Zn and C...
A:
Q: Draw a detailed free energy diagram comparing the exergonic Sy2 reactions with methoxide and ethanol...
A: Here we have to draw free energy diagram comparing the exogeneric SN2 reaction with methoxide ion an...
Q: The sulfate ion level in a municipal water supply is given as 46.1 ppm. What is [SO42-] in this wate...
A: Answer: Concentration of sulfate ion given is 46.1ppm which means in 106g solution, 46.1g sulfate io...
Q: polythene, Polyvinyl chloride and nylon are 3 examples of polymers have been given choose further 3 ...
A: A polymer is a large molecule or macromolecules which essentially is a combination of many monomers...
Q: CH3-CH2-C-o-CH3 butanone
A:
Q: 0.15- 0.05 0.02 0.04 0.06 0.08 t (s) Jse this graph to answer the following questions: What is the h...
A: Decomposition of ammonia to form hydrogen and nitrogen gas is a first order reaction. This means tha...
Q: 26. What would be the new pH of a 1L buffer solution (pH=7.8) upon the addition of 3mL 0.0001 M NaOH...
A: Answer: Buffer solution is the type of solution that resist the change in its pH on adding the small...
Q: 6. Give the MAJOR product formed when each of the following alcohols is heated in the presence of co...
A: Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for yo...
Q: 26. A molecular orbital can contain how many electrons? A. One B. Two C. Three D. Four 27. It is the...
A:
Q: A reaction A(aq)+B(aq) = C(aq) has a standard free-energy change of –3.75 kJ/mol at 25 °C. What are ...
A:
Q: For this question, I would like to know how the diverse ion effect would affect the solubility of Ca...
A: To explain the diverse ion effect on the solubility of Ca(OH)2
Q: Calculate the normality of a 22.5mL sample of H2SO4 if the volume of 1.1055N NaOH used in the standa...
A: Given, Volume of H2SO4 = 22.5 mL Molarity of NaOH = 1.1055 N Volume of NaOH = 20.70 mL
Q: Identify the reactant that gets oxidized in the following reaction. 2IO3- (aq) +10CIO2- (aq) + 12H+ ...
A: Given reaction , 2IO3- + 10ClO2- + 12H+ ---> I2 + 10ClO2 + 6H2O We have to determine oxidation s...
Q: The equilibrium constant, K, for the following reaction is 6.30 at 723K. 2NH3(g) N2(g) + 3H2(g) If a...
A: [NH3] eqm = 0.267 mol /19.2 L = 0.0139 M [N2] eqm = 0.342 mol /19.2 L = 0.0178 M
Q: Water has a vapor pressure of 18.0 torr and a density of 0.997 g/mL. Acetone (CHCOCH:) has a vapor p...
A:
Q: The oxidation number of a phosphorus atom in Pa is
A: Given : phosphorus
Q: Chemistry What does a melting point range tells us about the purity of a product?
A: Melting point:-The temperature at which the substances chenges from a solid to a liquid form, this p...
Q: Based on Hume-Rothery's conditions, which of the following systems would be expected to display unli...
A: Since you have asked multiple questions questions, we will solve the first four question for you. If...
Q: how you determine the pH when 15 ml of 0.100 M HCI is ado 080 M NH3 (K, = 1.6 x 10-6)
A: Given, Kb = 1.6×10-6 pKb = -log(Kb) = -log(1.6×10-6) = 5.82 Molarity of HCl = 0.100 M Volume of HCl...
Q: A 0.450 M solution of KCI needs to be prepared through dilution. A 2.00 M stock solution will be add...
A: 0.450M solution of KCl is to be prepared, Let it be M1 = 0.450 M 2.0 M of stock solution , Let it wi...
Q: Part II. Arrange the following compounds in order of increasing basicity. 7. NazO, Rb2O, K2O 8. Ga(O...
A: Ans. Part II Arrangement of the given compounds in order of increasing basicity: (7) Na2O < K2...
Q: weak acids
A: Ka is a dissociation constant of weak acid ie. HA======H+ +A- on the titration of acid with...
Q: Why is it easier to see all three pKa values in a tripolic acid but not all 3 equivalence points?
A:
Q: 3. What is the molar concentration of nitric acid (63.0 g/mol) in a solution that is 68.10 % HNO3 (w...
A: Given that, Molar mass of HNO3 = 63.0 g/mol percentage of composition of solution = 68.10 % Specific...
Q: 1.Can the states of matter change from one form to another (ex solid to liquid)? Yes or No? How? 2....
A: Answer: Matter exists in three physical states: 1. Solid state 2. Liquid state 3. Gaseous state
Q: How to identify constitutional isomers from stereoisomers? Can you provide an example? With molecula...
A: Stereochemistry is branch of chemistry in which we deal with arrangement of atoms in molecules.
Q: 7 A TITRATON curVE HAS AN EQUIVALENCE POINT AT pH =5.79 WHAT WAS THE UNKNOWN IN THE TITTATION, AND W...
A: Answer: This is an acid-base titration in which pH at the equivalence point is 5.79
Q: 3. The equilibrium constant for the reaction: N2 (g) +Oztg) is 2.60 x 103 at 1100 °C. If 0.820 mole ...
A:
Q: 10. According to the background information the Group III reagent is to be buffered at pH 10 to proo...
A: Answer- Data given- Fe3+ concentration is 0.1 N, ksp for Fe(OH)3 is 4.0x 10-38.
Q: Consider the following reaction occurring at a certain temperature: CO2(g) + H2(g) = CO(g) + H20(g) ...
A:
Q: If a 50.0g sample of Cu is heated to 180.00C and is placed in 100.0 ml of water in a calorimeter at ...
A: Given : Mass of Cu sample = 50.00 gm Initial temperature of Cu = 180 °C Volume of water = 100 ml ...
Q: MULTIPLE CHOICE PROBLEM SOLVING The boiling point of 350mL of substance A was determined to be 97.5o...
A:
Q: A 12.1 Moles NaOH solution means there are 12.1 moles of NaOH in liter of solution. Preparation of 1...
A: Given : Moles of NaOH = 12.1 gm Also molar mass of NaOH = 40 gm/mole
Q: Which of the following represents the generic form of a single-displacement reaction? A) A + BX → AX...
A:
Q: Ethylene glycol, HOCH2 CH,OH, is used as antifreeze. It is produced from ethylene oxide, C2H4O, by t...
A:
Q: XeF4 is a square planar molecule that has 9 vibrationalmodes. How many degrees of freedom does this ...
A: Solution - According to the question - the degrees of freedom of a particle may be defined as the nu...
Q: ne rate c
A:
Q: is urine homogeneous or heterogeneous mixture?
A: A mixture is made of two or more substances that are combined but they are not combined chemically.
Q: 1. Predict the product/s of the given reactions: b. S. H2O2 а. 2 CH3SH 100 °C
A: First reaction is an example of oxidation reaction in presence of H2O2 . Second one is and example o...
Q: (a) Predict and explain whether the following reaction is spontaneous or not: The standard entropy (...
A:
Q: Identify the functional group CH3-C 0-CH2-CH2-CH3
A: This question belong to some basic concept of organic chemistry that is general organic chemistry. F...
Q: H2N' IZ
A: The objective of the question is to find the product when the given molecule treated with strong red...
Q: 2. How could you use the 4 (FOUR) forms of spectroscopy that we have discussed to give you an indica...
A:
PLEASE ANSWER TO YOUR BEST. i REALLY WANT TO MAKE SURE I GET THIS RIGHT. just need answer no need all steps
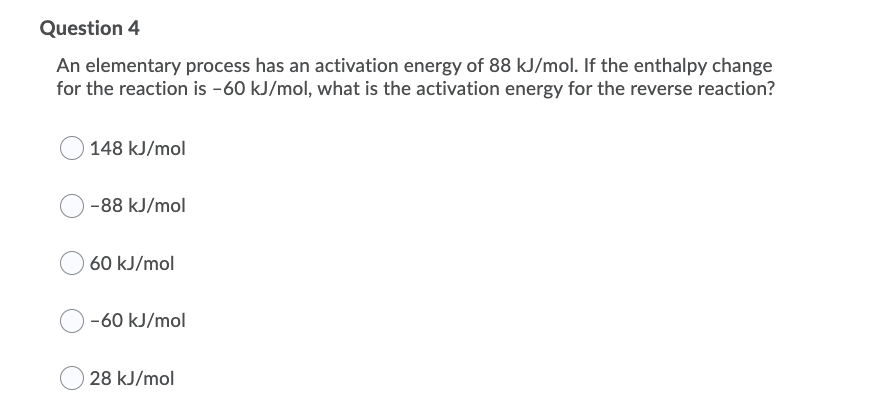
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- An elementary process has an activation energy of 40 kJ/mol. If the activation energy for the reverse reaction is 20 kJ/mol, what is the enthalpy change for the response? Question 4 options: –60 kJ/mol 20 kJ/mol 60 kJ/mol 40 kJ/mol –20 kJ/mo1. The activation energy Ea for a particular reaction is 50.0 kJ/mol. How much faster is the reaction at 313 K than at 310.0 K? (R = 8.314 J/mol • K) 2.An experimental plot of ln(k) vs. 1/T is obtained in lab for a reaction. The slope of the best-fit line for the graph is -3005 K. What is the value of the activation energy for the reaction in kJ/mol?QUESTION 6 Consider the reaction below at 100oC. T2U (g) -> 2 T (g) + U (g) When [T2U]o is 0.250 M, the reaction rate is 9.30 x 10-3 M/s, and when [T2U]o is 0.144 M, the reaction rate is 3.09 x 10-3 M/s. If the reaction has an activation energy of 66 kJ/mol, what is the numerical value of the rate constant at 25oC? Please assume k has the same units as those you selected for Question 3. A. 0.000703 B. 0.00472 C. 0.0373 D. 0.117
- Question 17 The rate constant for a certain reaction triples when the temperature increases from 300 K to 319 K. What is the activation ehergy of the reaction?An experimental plot of ln(k) vs. 1/T is obtained in lab for a reaction. The slope of the best fit line for the graph is -4750 K. What is the value of the activation energy for the reaction in kJ/mol?QUESTION 5 Consider the reaction below at 100oC. T2U (g) -> 2 T (g) + U (g) If given an initial reactant concentration [T2U]o = 0.250 M, what is [T] after 15 minutes have elapsed? The reaction rate is 9.30 x 10-3 M/s A. 0.486 M B. 0.243 M C. 0.00724 M D. 0.121 M
- What is the activation energy of the forward reaction? Question 15 options: 200 kJ 150 kJ 250 kJ 100 kJquestion 8: The rate of the following reaction was determined in three experiments. Using the data given below, determine the rate law for this reaction. You do not need to solve for the value of the rate constant k. 2 CO(g) + Cl2 (g) --> 2COCl (g) Experiment [CO]0 (M) [Cl2]0 (M) Rate (M.s-1) 1 0.030 0.050 1.68 x 10-4 2 0.015 0.050 1.72 x 10-4 3 0.015 0.100 3.56 x 10-4QUESTION 2 For the reaction...2 NO (g) + Cl 2(g) --> 2 NOCl (g)Given the following data, determine the order with respect to each reactantExp 1: [NO] = 0.10M, [Cl 2] = 0.20M, Rate (M/s) = 6.80 x 10 -6Exp 2 :[NO] = 0.10M, [Cl 2] = 0.40M, Rate (M/s) = 2.72 x 10 -5Exp. 3:[NO] = 0.20M, [Cl 2] = 0.40M, Rate (M/s) = 5.44 x 10 -5 a. The reaction is first order with respect to NO and first order with respect to Cl2. b. The reaction is second order with respect to NO and second order with respect to Cl2. c. The reaction is second order with respect to NO and first order with respect to Cl2. d. The reaction is zero order with respect to NO and first order with respect to Cl2. e. The reaction is first order with respect to NO and second order with respect to Cl2.
- A) Which of the following are the requirements of an effective collision between two or more molecules? Check all that apply. high temperature sufficient kinetic energy all of them correct orientation presence of a catalyst B) Which of the following would have the lowest rate of reaction? 50 °C, powdered Zinc metal, 5.0 M HCl 20 °C, powdered Zinc metal, 2.0 M HCl 20 °C, zinc metal, 1.0M HCl 50 °C, zinc metal, 5.0 M HClPlease help: A) The activation energy, Ea, for a particular reaction is 13.6 kJ/mol. If the rate constant at 475 K is 0.0450 1/min, then what is the value of the rate constant at 769 K? (R = 8.314 J/mol • K) B)The activation energy, Ea, for a particular reaction is 13.6 kJ/mol. If the rate constant at 754 °C is 24.5/min, at what temperature (in °C) will the rate constant be 18.5/min? (R = 8.314 J/mol • K) C)The activation energy, Ea, for a particular reaction is 19.4 kJ/mol. If the rate constant at 80 °C is 0.820 M⁻¹s⁻¹, then what is the value of the rate constant at 187 °C? (R = 8.314 J/mol • K) D)The activation energy, Ea, for a particular reaction is 37.8 kJ/mol. If the rate constant at 280 K is 0.178 M/s, then what is the value of the rate constant at 449 K? (R = 8.314 J/mol • K) Thank you so much!The rate constant for a reaction at 25.0°C is 0.010 s-1 and its EA is 35.8 kJ. Find the rateconstant at 50.0°C(A) 0.021 s-1(B) 0.0033 s-1(C) 0.010 s-1(D) 0.031 s-1

