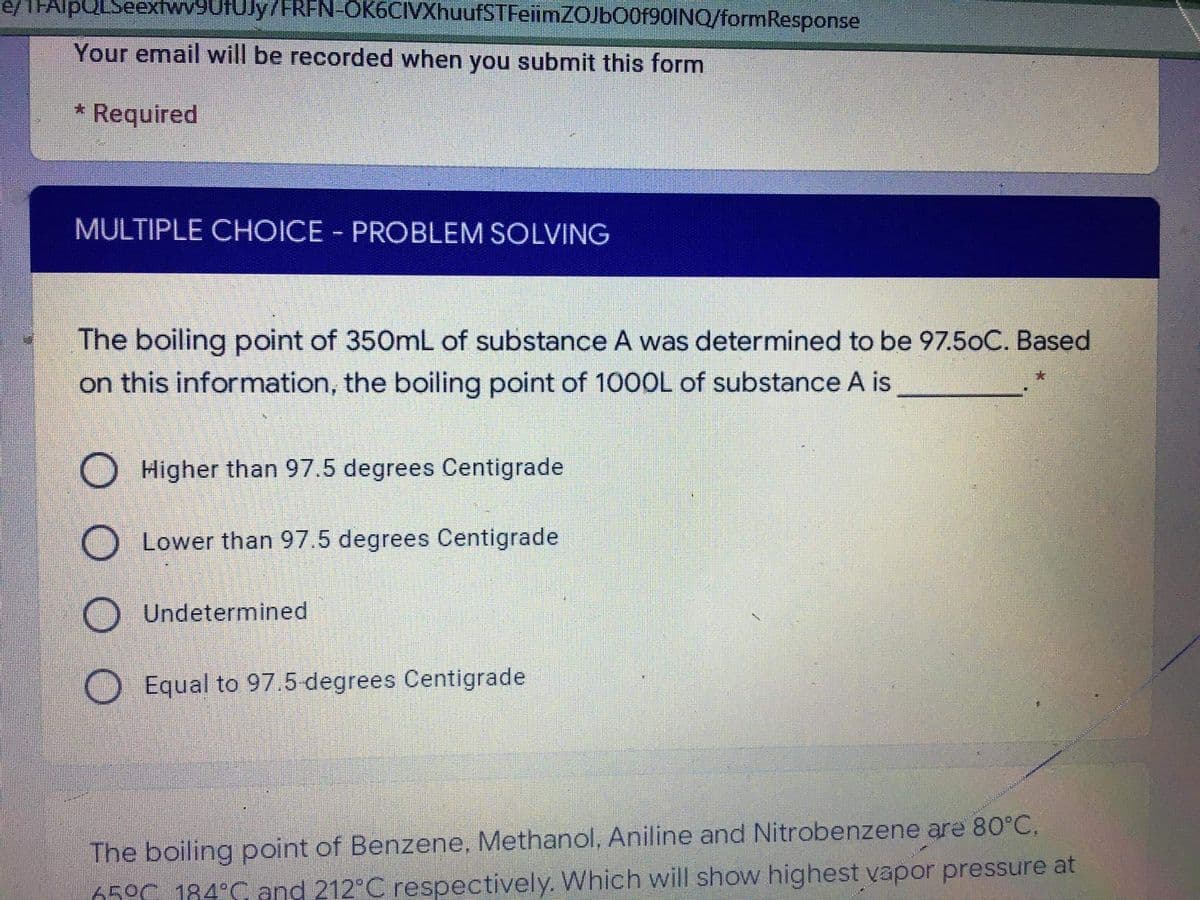
MULTIPLE CHOICE PROBLEM SOLVING The boiling point of 350mL of substance A was determined to be 97.5oC. Based on this information, the boiling point of 1000L of substance A is O Higher than 97.5 degrees Centigrade O Lower than 97.5 degrees Centigrade O Undetermined O Equal to 97.5-degrees Centigrade
MULTIPLE CHOICE PROBLEM SOLVING The boiling point of 350mL of substance A was determined to be 97.5oC. Based on this information, the boiling point of 1000L of substance A is O Higher than 97.5 degrees Centigrade O Lower than 97.5 degrees Centigrade O Undetermined O Equal to 97.5-degrees Centigrade
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 67QAP
Related questions
Question
Transcribed Image Text:e/1FAlpQLSeextwy9UfUly7FREN-OK6CIVXhuufSTFeiimZOJb00f90INQ/formResponse
Your email will be recorded when you submit this form
* Required
MULTIPLE CHOICE PROBLEM SOLVING
|3|
The boiling point of 350mL of substance A was determined to be 97.5oC. Based
on this information, the boiling point of 100OL of substance A is
Higher than 97.5 degrees Centigrade
Lower than 97.5 degrees Centigrade
Undetermined
Equal to 97.5 degrees Centigrade
The boiling point of Benzene, Methanol, Aniline and Nitrobenzene are 80°C,
650C 184°C and 212 C respectively. Which will show highest vapor pressure at
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning