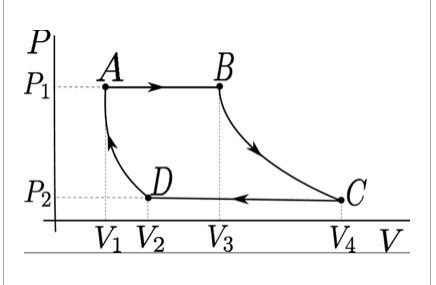
An ideal gas follows the thermodynamic path shown in the figure. From "A" to "B" the process is at constant pressure and 140kJ of heat flows into the system. From "B" to "C" the process is isothermal. From "C" to "D" the process is at constant pressure and 200kJ of heat flows out of the system. From "D" to "A" the process is adiabatic. Estimate the change in internal energy from "D" to "A" P 1 = 300,000 Pa P 2 = 100,000 V1 = 0.09m^3 V2 = 0.2m^3 V3 = 0.4m^3 V4 = 1.2m^3
An ideal gas follows the thermodynamic path shown in the figure. From "A" to "B" the process is at constant pressure and 140kJ of heat flows into the system. From "B" to "C" the process is isothermal. From "C" to "D" the process is at constant pressure and 200kJ of heat flows out of the system. From "D" to "A" the process is adiabatic. Estimate the change in internal energy from "D" to "A" P 1 = 300,000 Pa P 2 = 100,000 V1 = 0.09m^3 V2 = 0.2m^3 V3 = 0.4m^3 V4 = 1.2m^3
University Physics Volume 1
18th Edition
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:William Moebs, Samuel J. Ling, Jeff Sanny
Chapter2: Vectors
Section: Chapter Questions
Problem 21CQ: What is wrong with the following expressions? How can you correct them? (a) C=AB , (b) C=AB , (c)...
Related questions
Question
An ideal gas follows the
Estimate the change in internal energy from "D" to "A"
P 1 = 300,000 Pa
P 2 = 100,000
V1 = 0.09m^3
V2 = 0.2m^3
V3 = 0.4m^3
V4 = 1.2m^3
Transcribed Image Text:P|
A
В
P2
Vị V2 V3
VẠ V.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University