An insulated Thermos contains 190 cm³ of hot coffee at 92.0°C. You put in a 12.0 g ice cube at its melting point to cool the coffee. By how many degrees has your coffee cooled once the ice has melted and equilibrium is reached? Treat the coffee as though it were pure water and neglect energy exchanges with the environment. The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg. The density of water is 1.00 g/cm³.
An insulated Thermos contains 190 cm³ of hot coffee at 92.0°C. You put in a 12.0 g ice cube at its melting point to cool the coffee. By how many degrees has your coffee cooled once the ice has melted and equilibrium is reached? Treat the coffee as though it were pure water and neglect energy exchanges with the environment. The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg. The density of water is 1.00 g/cm³.
Related questions
Question
Final Answer must be a decimal or whole numbers only!!!
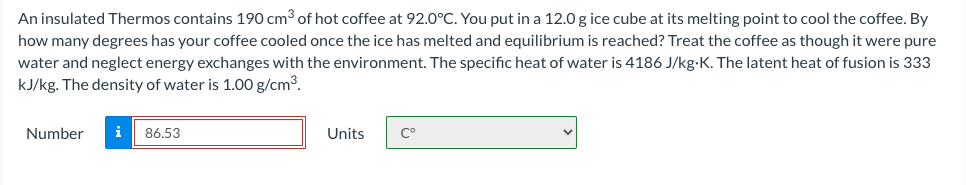
Transcribed Image Text:An insulated Thermos contains 190 cm³ of hot coffee at 92.0°C. You put in a 12.0 g ice cube at its melting point to cool the coffee. By
how many degrees has your coffee cooled once the ice has melted and equilibrium is reached? Treat the coffee as though it were pure
water and neglect energy exchanges with the environment. The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333
kJ/kg. The density of water is 1.00 g/cm3.
Number
i
86.53
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images
