An sp hybridizedc-CI bond is more polar than an sp? hybridized C- CI bond. (a) Explain why this phenomenon arises. (b) Rank the following compounds in order of increasing boiling point. Br .CI
An sp hybridizedc-CI bond is more polar than an sp? hybridized C- CI bond. (a) Explain why this phenomenon arises. (b) Rank the following compounds in order of increasing boiling point. Br .CI
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter7: Cycloalkanes
Section: Chapter Questions
Problem 9CTQ: a model of cyclohexane in a chair conformation, and explain why the names “axial” and“equatorial’...
Related questions
Question
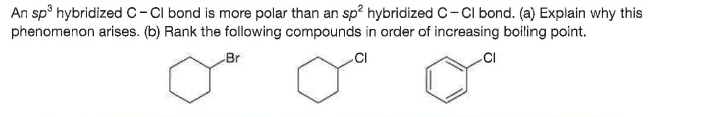
Transcribed Image Text:An sp hybridizedc-CI bond is more polar than an sp? hybridized C- CI bond. (a) Explain why this
phenomenon arises. (b) Rank the following compounds in order of increasing boiling point.
Br
.CI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning