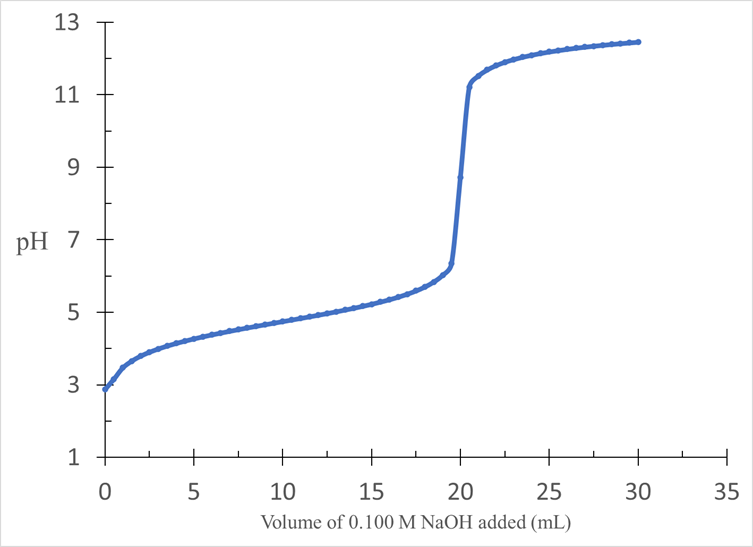
An unknown acid is dissolved in 25.0 mL of water and titrated with 0.100 M NaOH. The results are shown in the titration curve above. Determine the molar mass of the acid if 0.200 g were dissolved.
An unknown acid is dissolved in 25.0 mL of water and titrated with 0.100 M NaOH. The results are shown in the titration curve above. Determine the molar mass of the acid if 0.200 g were dissolved.
Chapter5: Errors In Chemical Analyses
Section: Chapter Questions
Problem 5.10QAP
Related questions
Question
100%
An unknown acid is dissolved in 25.0 mL of water and titrated with 0.100 M NaOH. The results are shown in the titration curve above. Determine the molar mass of the acid if 0.200 g were dissolved.
Transcribed Image Text:13
11
pH 7
5
1
+
5
10
15
20
25
30
35
Volume of 0.100 M NaOH added (mL)
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning