+ Titration of Strong Acid with Strong Base 100. mL of 0.200 M HCI is titrated with 0.250 M NaOH. A titration involves adding a reactant of known quantity to a solution of an another reactant while monitoring the equilibrium concentrations. This allows one to determine the concentration of the second reactant. A pH titration curve specifically monitors the pH as a function of the titrant. Part A What is the pH of the solution after 50.0 mL of base has been added? When conducting calculations involving a titration, the first step is to write the balanced chemical equation. Then, use the stoichiometric ratios developed from this equation to determine how many moles of each reagent are reacting. Express the pH numerically. > View Available Hint(s)
+ Titration of Strong Acid with Strong Base 100. mL of 0.200 M HCI is titrated with 0.250 M NaOH. A titration involves adding a reactant of known quantity to a solution of an another reactant while monitoring the equilibrium concentrations. This allows one to determine the concentration of the second reactant. A pH titration curve specifically monitors the pH as a function of the titrant. Part A What is the pH of the solution after 50.0 mL of base has been added? When conducting calculations involving a titration, the first step is to write the balanced chemical equation. Then, use the stoichiometric ratios developed from this equation to determine how many moles of each reagent are reacting. Express the pH numerically. > View Available Hint(s)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 50QAP: Morphine, C17H19O3N, is a weak base (K b =7.4107). Consider its titration with hydrochloric acid. In...
Related questions
Question
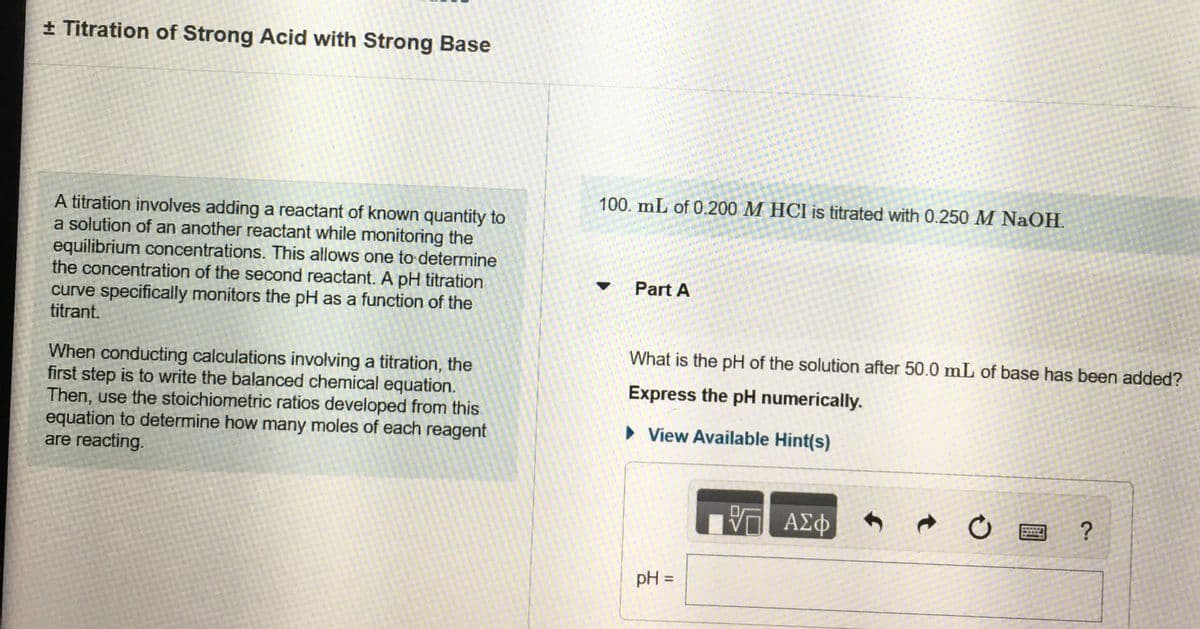
Transcribed Image Text:+ Titration of Strong Acid with Strong Base
100. mL of 0.200 M HCl is titrated with 0.250 M NaOH.
A titration involves adding a reactant of known quantity to
a solution of an another reactant while monitoring the
equilibrium concentrations. This allows one to determine
the concentration of the second reactant. A pH titration
curve specifically monitors the pH as a function of the
titrant.
Part A
What is the pH of the solution after 50.0 mL of base has been added?
When conducting calculations involving a titration, the
first step is to write the balanced chemical equation.
Then, use the stoichiometric ratios developed from this
equation to determine how many moles of each reagent
are reacting.
Express the pH numerically.
• View Available Hint(s)
ΑΣφ
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning