An unknown sample containing moved alkal (NaOH, NaHCO or Na CO) was analyzed using the double flask method. A 250 mg sample was dissolved in 250 m CO₂ free water. A 20.0 ml aliquot of this sample required 11.3 ml. of 0.009125 MHCI solution to reach the phenolphthalein end point. Another 29.0 ml aliquot of the sample was titrated to the bromocresol green endpoint uning 311 mt of the standard acid. How many millimoles of the components are there in the original solid sample) O028 mmol Nejco 00103 mmol NaOH 0.078 mm Na Co O Cannot be determined 01:29 ml NaCO, 0.97 minol NaHCO, 129 mmol NaOH, 097 inmol NaO
An unknown sample containing moved alkal (NaOH, NaHCO or Na CO) was analyzed using the double flask method. A 250 mg sample was dissolved in 250 m CO₂ free water. A 20.0 ml aliquot of this sample required 11.3 ml. of 0.009125 MHCI solution to reach the phenolphthalein end point. Another 29.0 ml aliquot of the sample was titrated to the bromocresol green endpoint uning 311 mt of the standard acid. How many millimoles of the components are there in the original solid sample) O028 mmol Nejco 00103 mmol NaOH 0.078 mm Na Co O Cannot be determined 01:29 ml NaCO, 0.97 minol NaHCO, 129 mmol NaOH, 097 inmol NaO
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter24: The Standardization Of A Basic Solution And The Determination Of The Molar Mass Of An Acid
Section: Chapter Questions
Problem 3ASA: A 0.3012g sample of an unknown monoprotic acid requires 24.13mL of 0.0944MNaOH for neutralization to...
Related questions
Question
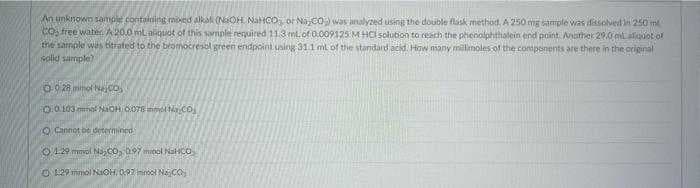
Transcribed Image Text:An unknown sample containing mixed alkal (NaOH NaHCO or Na,CO) was analyzed using the double flask method, A 250 mg sample was dissolved in 250 m
CO3 free water. A 20.0 mL aliquot of this sample required 11.3 ml. of 0.009125 M HCl solution to reach the phenolphthalein end point. Another 29.0 ml aliquot of
the sample was bitrated to the bromocresol green endpoint using 31.1 ml of the standard acid. How many millimoles of the components are there in the original
solid sample)
0-928 mmol No.
0.0103 mmol NaOH 0078 m N.CO
Cannot be determined
012 mol NaC0, 197 minol NaHCO
129 mmol NaOH, 097ml Najco
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning