an you demonstrate the process path for enthalpy 2 (going out)?
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Can you demonstrate the process path for enthalpy 2 (going out)?
![||!
PDF *Elementary Principles of Chemic X PDF *Elementary Principles of Chemic X +
O
56°F
Clear
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
Draw
(T) Read aloud
Q Search
out
3. Choose reference states for acetone and nitrogen.
Substance
428 of 695
The reference states may be chosen for computational convenience, since the choice has no
effect on the calculated value of AH. We will arbitrarily choose the inlet stream condition for
nitrogen (65°C, 1 atm) as the reference state for this species and one of the two outlet stream
conditions for acetone (1, 20°C, 5 atm) as the reference state for acetone, which will enable us to set
the corresponding values for Ĥ in the enthalpy table equal to zero instead of having to calculate them.
4. Construct an inlet-outlet enthalpy table.
We first write the chosen reference states, then construct the table shown below:
References: Ac(1, 20°C, 5 atm), N₂(g, 65°C, 1 atm)
nin
Ĥ in
(mol/s) (kJ/mol)
66.9 Ĥ₁
Ac(v)
Ac(1)
N₂
33.1
in
0
nout
(mol/s)
3.35
63.55
33.1
Ĥ out
(kJ/mol)
Note the following points about the table:
• Nitrogen has only one inlet state (gas, 65°C, 1 atm) and one outlet state (gas, 20°C, 5 atm), so we
need only one row in the table for N₂. Acetone has one inlet state (vapor, 65°C, 1 atm) but two
outlet states (vapor and liquid, each at 20°C and 5 atm), so we need two rows for this species.
■
Ĥ₂
0
Ĥ3
• We mark out (using dashes) the two cells corresponding to in and Hin for liquid acetone, since no
liquid acetone enters the system.
• The è values are obtained from the flowchart. The flow rate of acetone vapor at the inlet, for
example, is determined as (100 mol/s)[0.669 mol Ac(v)/mol] = 66.9 mol Ac(v)/s.
• Since the nitrogen entering the system and the liquid acetone leaving the system area are at their
reference states, we set their specific enthalpies equal to zero.
H
• Three unknown specific enthalpies have been labeled and must be determined in Step 5.
5. Calculate all unknown specific enthalpies.
To calculate the three unknown specific enthalpies in the table, we construct hypothetical
process paths from the reference states to the states of the species in the process and evaluate AĤ for
each path. This is the part of the calculation you have not yet learned to do. We will show you the
calculation of H₁ to illustrate the method, give the results of the other calculations, and go into detail
about the required procedures in Sections 8.2-8.5.
Ĥ₁ = specific enthalpy of Ac(v, 65°C, 1 atm) relative to Ac(1, 20°C, 5 atm)
= AĤ for Ac(1, 20°C, 5 atm) → Ac(v, 65°C, 1 atm)
When choosing a process path for the determination of AH, it helps to know that formulas and
data are given in this chapter for enthalpy changes corresponding to certain types of processes:
• Section 8.2 gives the formula AĤ = VAP for a change in pressure (AP) undergone by a liquid or
solid with constant specific volume V. The value of V for liquid acetone may be determined as
0.0734 L/mol from the specific gravity (0.791) given in Table B.1.
{"
@
683
ENG
Sign in
00
:
11:16 PM
5/23/2023](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F9ba88ecd-654b-4f6b-9783-e93c33ca6a16%2F48d1532b-3d37-43e5-b1bb-bbe0251c5baa%2F8q6hd9_processed.png&w=3840&q=75)
Transcribed Image Text:||!
PDF *Elementary Principles of Chemic X PDF *Elementary Principles of Chemic X +
O
56°F
Clear
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
Draw
(T) Read aloud
Q Search
out
3. Choose reference states for acetone and nitrogen.
Substance
428 of 695
The reference states may be chosen for computational convenience, since the choice has no
effect on the calculated value of AH. We will arbitrarily choose the inlet stream condition for
nitrogen (65°C, 1 atm) as the reference state for this species and one of the two outlet stream
conditions for acetone (1, 20°C, 5 atm) as the reference state for acetone, which will enable us to set
the corresponding values for Ĥ in the enthalpy table equal to zero instead of having to calculate them.
4. Construct an inlet-outlet enthalpy table.
We first write the chosen reference states, then construct the table shown below:
References: Ac(1, 20°C, 5 atm), N₂(g, 65°C, 1 atm)
nin
Ĥ in
(mol/s) (kJ/mol)
66.9 Ĥ₁
Ac(v)
Ac(1)
N₂
33.1
in
0
nout
(mol/s)
3.35
63.55
33.1
Ĥ out
(kJ/mol)
Note the following points about the table:
• Nitrogen has only one inlet state (gas, 65°C, 1 atm) and one outlet state (gas, 20°C, 5 atm), so we
need only one row in the table for N₂. Acetone has one inlet state (vapor, 65°C, 1 atm) but two
outlet states (vapor and liquid, each at 20°C and 5 atm), so we need two rows for this species.
■
Ĥ₂
0
Ĥ3
• We mark out (using dashes) the two cells corresponding to in and Hin for liquid acetone, since no
liquid acetone enters the system.
• The è values are obtained from the flowchart. The flow rate of acetone vapor at the inlet, for
example, is determined as (100 mol/s)[0.669 mol Ac(v)/mol] = 66.9 mol Ac(v)/s.
• Since the nitrogen entering the system and the liquid acetone leaving the system area are at their
reference states, we set their specific enthalpies equal to zero.
H
• Three unknown specific enthalpies have been labeled and must be determined in Step 5.
5. Calculate all unknown specific enthalpies.
To calculate the three unknown specific enthalpies in the table, we construct hypothetical
process paths from the reference states to the states of the species in the process and evaluate AĤ for
each path. This is the part of the calculation you have not yet learned to do. We will show you the
calculation of H₁ to illustrate the method, give the results of the other calculations, and go into detail
about the required procedures in Sections 8.2-8.5.
Ĥ₁ = specific enthalpy of Ac(v, 65°C, 1 atm) relative to Ac(1, 20°C, 5 atm)
= AĤ for Ac(1, 20°C, 5 atm) → Ac(v, 65°C, 1 atm)
When choosing a process path for the determination of AH, it helps to know that formulas and
data are given in this chapter for enthalpy changes corresponding to certain types of processes:
• Section 8.2 gives the formula AĤ = VAP for a change in pressure (AP) undergone by a liquid or
solid with constant specific volume V. The value of V for liquid acetone may be determined as
0.0734 L/mol from the specific gravity (0.791) given in Table B.1.
{"
@
683
ENG
Sign in
00
:
11:16 PM
5/23/2023
Transcribed Image Text:64°F
Clear
Elementary Principles of Chemica X +
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
Draw
(T)
Read aloud
Example 8.1-1
Equipment Encyclopedia
condenser
www.wiley.com/college/felder
nixture
compounds
prodiems.
(8.15
408 CHAPTER 8 Balances on Nonreactive Processes
Q Search
428 of 695
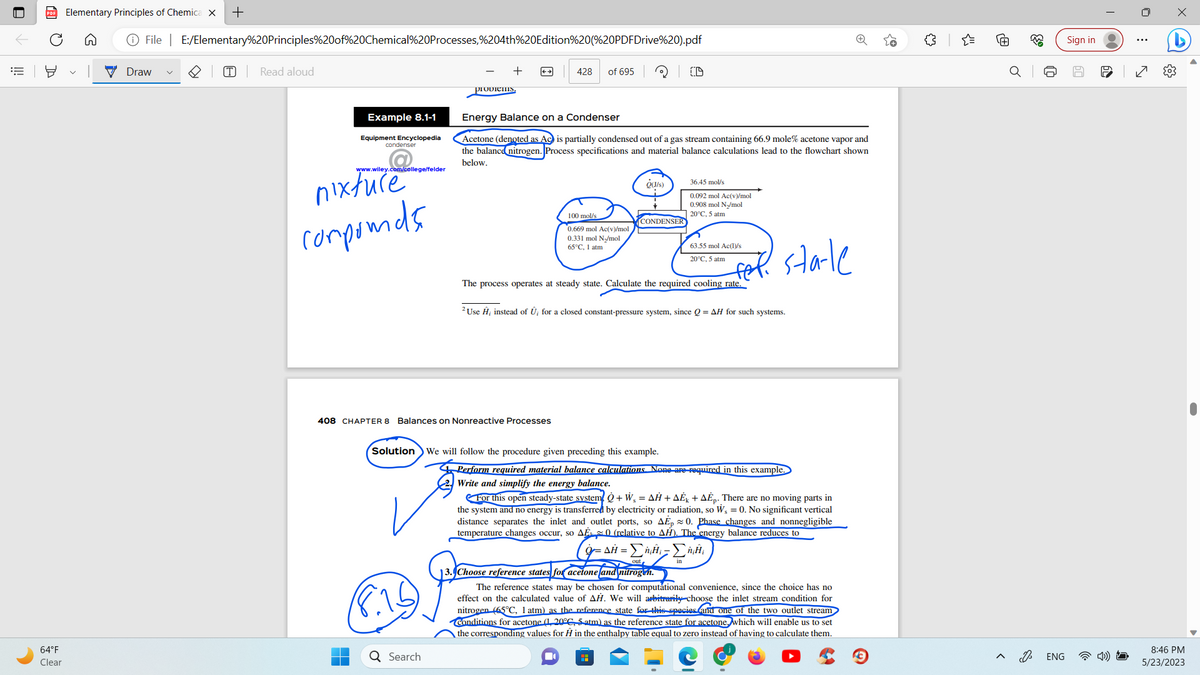
Energy Balance on a Condenser
Acetone (denoted as Ac is partially condensed out of a gas stream containing 66.9 mole% acetone vapor and
the balance nitrogen. Process specifications and material balance calculations lead to the flowchart shown
below.
100 mol/s
0.669 mol Ac(v)/mol
0.331 mol N₂/mol
65°C, 1 atm
O(J/s)
CONDENSER
Solution We will follow the procedure given preceding this example.
36.45 mol/s
0.092 mol Ac(v)/mol
0.908 mol N₂/mol
20°C, 5 atm
63.55 mol Ac(1)/s
20°C, 5 atm
The process operates at steady state. Calculate the required cooling rate.
2 Use Ĥ; instead of Û, for a closed constant-pressure system, since Q=AH for such systems.
sta-te
Perform required material balance calculations. None are required in this example.
Write and simplify the energy balance.
For this open steady-state system + W₁ = AH+AE+AEp. There are no moving parts in
the system and no energy is transferred by electricity or radiation, so W₁ = 0. No significant vertical
distance separates the inlet and outlet ports, so AEp 0. Phase changes and nonnegligible
temperature changes occur, so AE 0 (relative to AH). The energy balance reduces to
Ο =ΔΗ - ΣΗ-ΣΗ
13. Choose reference states for acetone and nitrogen.
The reference states may be chosen for computational convenience, since the choice has no
effect on the calculated value of AH. We will arbitrarily choose the inlet stream condition for
nitrogen (65°C, 1 atm) as the reference state for this species and one of the two outlet stream
Conditions for acetone (1, 20°C, 5 atm) as the reference state for acetone, which will enable us to set
the corresponding values for H in the enthalpy table equal to zero instead of having to calculate them.
{"
@
683
D
ENG
Sign in
00
60
:
8:46 PM
5/23/2023
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The