Analysis: 1. 2. 3. Trial number lighter number initial mass of lighter final mass of lighter (we'll get this after they have dried overnight) Temperature (read from thermometer) volume of butane (read from eudiometer tube) Barometric pressure (read from barometer) vapor pressure of water (read from chart of vapor pressures) 1 13.35099 13.24820 22.4°C 4482 MAL 29.85mg 20.45 2 3 MO Convert the barometric pressure from inHg to mmHg. (1 in = 25.4 mm) Use Dalton's Law to calculate pressure of the butane alone. Using the Ideal Gas Law, calculate the moles of butane for each trial using the ideal gas law.
Analysis: 1. 2. 3. Trial number lighter number initial mass of lighter final mass of lighter (we'll get this after they have dried overnight) Temperature (read from thermometer) volume of butane (read from eudiometer tube) Barometric pressure (read from barometer) vapor pressure of water (read from chart of vapor pressures) 1 13.35099 13.24820 22.4°C 4482 MAL 29.85mg 20.45 2 3 MO Convert the barometric pressure from inHg to mmHg. (1 in = 25.4 mm) Use Dalton's Law to calculate pressure of the butane alone. Using the Ideal Gas Law, calculate the moles of butane for each trial using the ideal gas law.
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 8P
Related questions
Question
100%
How would you answer the third question?
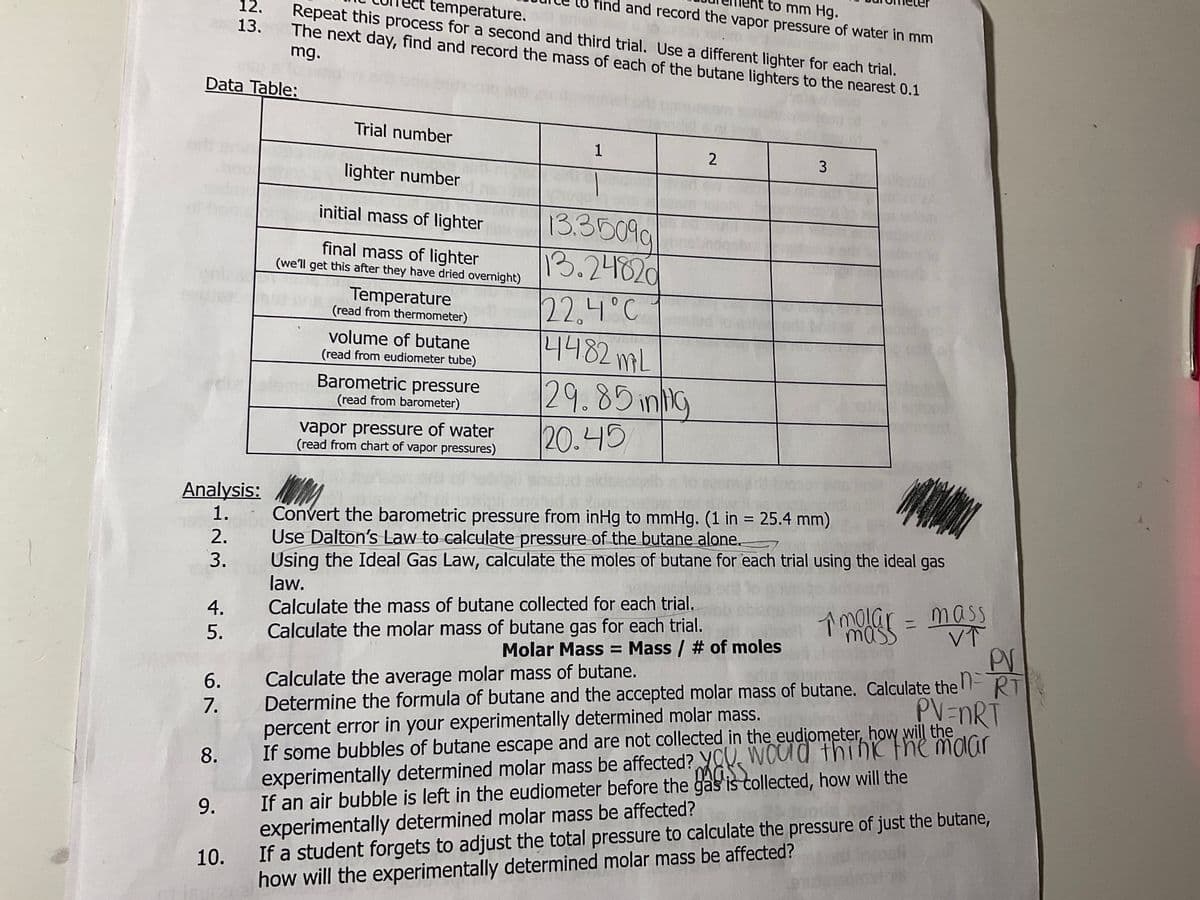
Transcribed Image Text:Data Table:
Analysis:
1.
2.
3.
4.
5.
6.
7.
8.
12.
13.
9.
10.
ct temperature.
Repeat this process for a second and third trial. Use a different lighter for each trial.
The next day, find and record the mass of each of the butane lighters to the nearest 0.1
mg.
Trial number
lighter number
initial mass of lighter
final mass of lighter
(we'll get this after they have dried overnight)
Temperature
(read from thermometer)
volume of butane
(read from eudiometer tube)
Barometric pressure
(read from barometer)
vapor pressure of water
(read from chart of vapor pressures)
1
to mm Hg.
nd record the vapor pressure of water in mm
13.3509g
13.24820
22.4°C
4482mL
29.85 ing
20.45
2
3
Convert the barometric pressure from inHg to mmHg. (1 in = 25.4 mm)
Use Dalton's Law to calculate pressure of the butane alone.
Using the Ideal Gas Law, calculate the moles of butane for each trial using the ideal gas
law.
Calculate the mass of butane collected for each trial.
Calculate the molar mass of butane gas for each trial.
1 molar = mass
PV
mass
Molar Mass = Mass / # of moles
Calculate the average molar mass of butane.
Determine the formula of butane and the accepted molar mass of butane. Calculate the RT
percent error in your experimentally determined molar mass.
PV=nRT
would think the malar
If some bubbles of butane escape and are not collected in the eudiometer, how will the
experimentally determined molar mass be affected?
If an air bubble is left in the eudiometer before the
experimentally determined molar mass be affected?
If a student forgets to adjust the total pressure to calculate the pressure of just the butane,
how will the experimentally determined molar mass be affected?
gas is collected, how will the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

