Analysis & Discussion: 1. Based on your three trials, discuss the precision of your data. 2. The actual concentration of the unknown is 0.200 M. (Check with instructor to ensure that this value is accurate.) Calculate the percent error based on the average molarity of your three trials. 3. Based on your answer to (2), discuss the accuracy of your data.
Analysis & Discussion: 1. Based on your three trials, discuss the precision of your data. 2. The actual concentration of the unknown is 0.200 M. (Check with instructor to ensure that this value is accurate.) Calculate the percent error based on the average molarity of your three trials. 3. Based on your answer to (2), discuss the accuracy of your data.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter15: Additional Aqueous Equilibria
Section: Chapter Questions
Problem 34QRT
Related questions
Question
Based on the data given answer the questions. Please ??
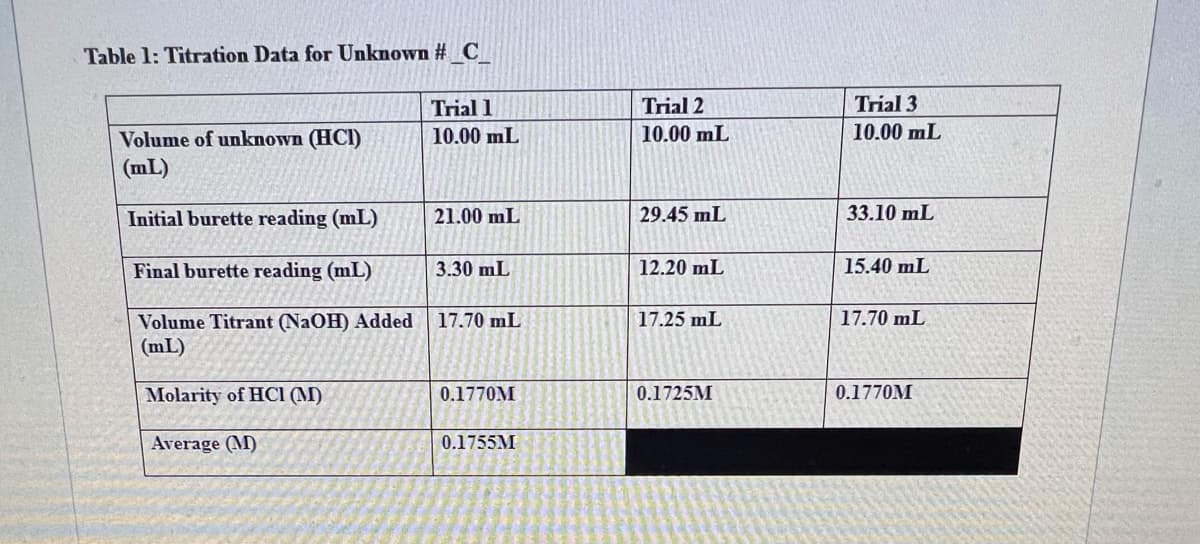
Transcribed Image Text:Table 1: Titration Data for Unknown #_C
Volume of unknown (HCI)
(mL)
Initial burette reading (mL)
Final burette reading (mL)
Volume Titrant (NaOH) Added
(mL)
Molarity of HCI (M)
Average (M)
Trial 1
10.00 mL
21.00 mL
3.30 mL
17.70 mL
0.1770M
0.1755M
Trial 2
10.00 mL
29.45 mL
12.20 mL
17.25 mL
0.1725M
Trial 3
10.00 mL
33.10 mL
15.40 mL
17.70 mL
0.1770M

Transcribed Image Text:Analysis & Discussion:
1. Based on your three trials, discuss the precision of your data.
2. The actual concentration of the unknown is 0.200 M. (Check with instructor to ensure that this value is
accurate.) Calculate the percent error based on the average molarity of your three trials.
3. Based on your answer to (2), discuss the accuracy of your data.
4. Discuss the potential sources of error that could have affected either/both your accuracy and precision.
(Examples could include items such as: a) You forgot to rinse the burette with the NaOH solution; b)
Bubbles of air were trapped in the burrette tip; c) You added 10 drops of phenolphthalein instead of 5
drops; d) The meniscus of NaOH was sitting right on the 32 line so you reported the reading as 32 mL
instead of 32.00 mL.) Explain HOW and WHY each of these may have impacted your results.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co