Analysis of bleach involves two sequential redox reactions: First, bleach is reacted in acid solution with excess iodide anion to produce yellow-colored lodine: Review I Constants I Periodic Table A sample of a new cleaning product, "Joe's Famous Bleach Cleaner," with a mass of 49.0g. was diluted with an acetic acid solution containing excess I.Asmall amount of starch indicator solution was then added, turning the solution a deep bluish-purple. The solution was then titrated with 0.260 M sodium thiosulfate, Na203, containing the lon S30s. A volume of 40.0 mL of sodium thiosulfate, the titrant, was needed to turn the solution coloriess. CIO+ 2H+ 2112+ C+ H20 Then, to determine how much of the lodine was formed, the solution is titrated with sodium thiosulfate solution: 12+ 252021+So Percent composition A bit of starch is added to the titration reaction. Starch is intensely blue in the presence of I2. The solution thus turns from deep blue to coloriess at the reaction equivalence point. To obtain percent composition by mass, use the equation given here: mass due to specific compone percent composition-tota o the mixture 100 • Part A What is the percentage composition by mass of NACIO in the bleach product? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) Tafnolat Ledo redo resat keyboard shortcuts belp 1.36 Submit Previous Answers
Analysis of bleach involves two sequential redox reactions: First, bleach is reacted in acid solution with excess iodide anion to produce yellow-colored lodine: Review I Constants I Periodic Table A sample of a new cleaning product, "Joe's Famous Bleach Cleaner," with a mass of 49.0g. was diluted with an acetic acid solution containing excess I.Asmall amount of starch indicator solution was then added, turning the solution a deep bluish-purple. The solution was then titrated with 0.260 M sodium thiosulfate, Na203, containing the lon S30s. A volume of 40.0 mL of sodium thiosulfate, the titrant, was needed to turn the solution coloriess. CIO+ 2H+ 2112+ C+ H20 Then, to determine how much of the lodine was formed, the solution is titrated with sodium thiosulfate solution: 12+ 252021+So Percent composition A bit of starch is added to the titration reaction. Starch is intensely blue in the presence of I2. The solution thus turns from deep blue to coloriess at the reaction equivalence point. To obtain percent composition by mass, use the equation given here: mass due to specific compone percent composition-tota o the mixture 100 • Part A What is the percentage composition by mass of NACIO in the bleach product? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) Tafnolat Ledo redo resat keyboard shortcuts belp 1.36 Submit Previous Answers
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.97QE: According to the Resource Conservation and Recovery Act (RCRA), waste material is classified as...
Related questions
Question
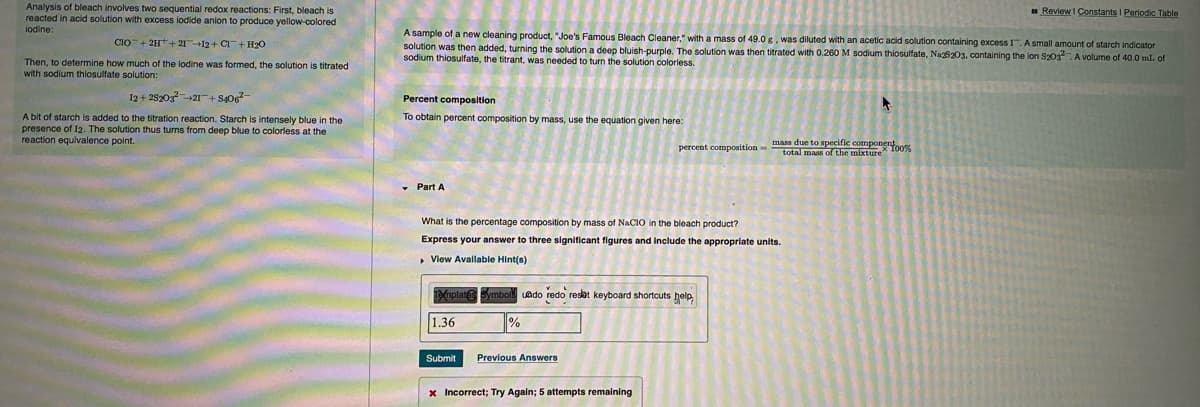
Transcribed Image Text:Analysis of bleach involves two sequential redox reactions: First, bleach is
reacted in acid solution with excess iodide anion to produce yellow-colored
Review I Constants I Periodic Table
lodine:
A sample of a new cleaning product, "Joe's Famous Bleach Cleaner," with a mass of 49.0 g, was diluted with an acetic acid solution containing excess I.A small amount of starch indicator
CIO+ 2H+2112+ CI+ H20
solution was then added, turning the solution a deep bluish-purple. The solution was then titrated with 0.260 M sodium thiosulfate, Na25203, containing the lon S203. A volume of 40.0 mL of
sodium thiosulfate, the titrant, was needed to turn the solution colorless.
Then, to determine how much of the lodine was formed, the solution is titrated
with sodium thiosulfate solution:
I2 + 282032I+ S40
Percent composition
A bit of starch is added to the titration reaction. Starch is intensely blue in the
presence of I2. The solution thus turns from deep blue to colorless at the
reaction equivalence point.
To obtain percent composition by mass, use the equation given here:
mass due to specific compone
percent composition-total mass of the mixture 100%
• Part A
What is the percentage composition by mass of NACIO in the bleach product?
Express your answer to three significant figures and include the appropriate units.
, View Available Hint(s)
Tafnplat mbol
uado redo resot keyboard shortcuts help
1.36
Submit
Previous Answers
x Incorrect; Try Again; 5 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning