ance the net ionic redox reaction. 2 Ca + 3 Sb3+ → 2 Ca2+ + 3 Sb Са + 2Sb'+ → 3 Ca2++Sb Са + Sb3+ Ca2++Sb 2 Ca + Sb3+ → Ca2+ + 3 Sb 3 Са + 2 Sb3+ → 3 Ca2+ + 2 Sb
ance the net ionic redox reaction. 2 Ca + 3 Sb3+ → 2 Ca2+ + 3 Sb Са + 2Sb'+ → 3 Ca2++Sb Са + Sb3+ Ca2++Sb 2 Ca + Sb3+ → Ca2+ + 3 Sb 3 Са + 2 Sb3+ → 3 Ca2+ + 2 Sb
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter19: The Chemistry Of The Main-group Elements
Section19.6: A Periodic Perspective: The Main-group Elements
Problem 19.8PSP
Related questions
Question
Need help with these homework questions about
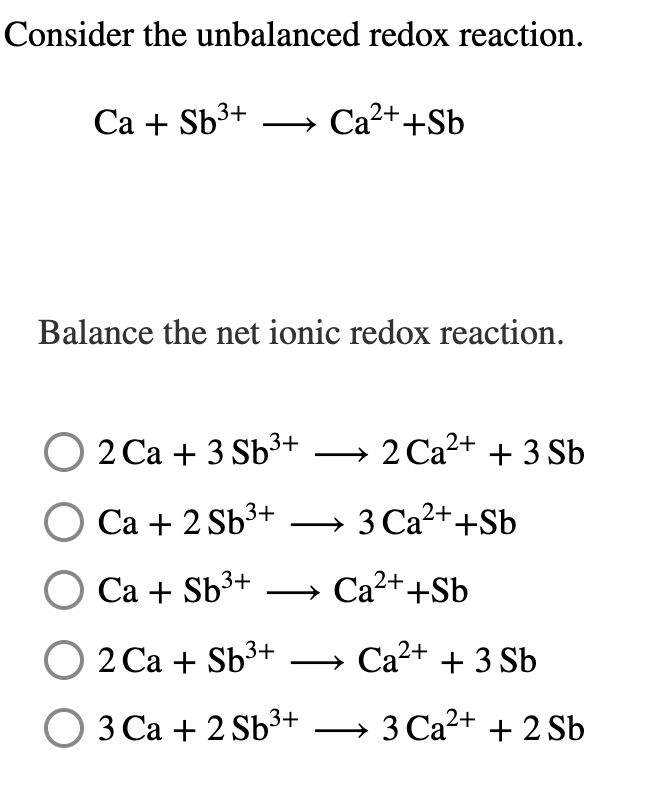
Transcribed Image Text:Consider the unbalanced redox reaction.
Са + Sb3+
» Ca2++Sb
Balance the net ionic redox reaction.
O 2 Ca + 3 Sb³+ → 2 Ca2+ + 3 Sb
|
Са + 2 Sb3+
3 Ca2++Sb
O Ca + Sb³+
» Ca2++Sb
|
О 2 Са + Sb3+
→ Ca2+ + 3 Sb
О З Са + 2 sb3+
3 Ca2+ + 2 Sb
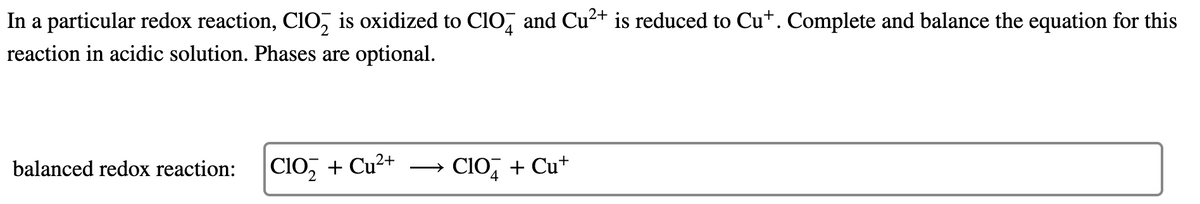
Transcribed Image Text:In a particular redox reaction, Cio, is oxidized to ClO, and Cu²+ is reduced to Cu+. Complete and balance the equation for this
reaction in acidic solution. Phases are optional.
Clo, + Cu²+
→ CIO, + Cu+
balanced redox reaction:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co
