Answer all three please ✓ 2 TABLE 13.2 Inorganic Compounds - Be sure to show the proper geometries! Species Lewis Dot Structure and Resonance structures including formal charge VSEPR Theory and Valence Bond Theory Classifications Molecular Geometry HCN Indicate the hybridization of both the C and N Total Valence Electrons SO₂ Show all reso- nance structures and formal charges. Total Valence Electrons AsCl₂ Are the Cl atoms equato- rial, axial, or both? Total Valence Electrons H-C-N bond angle Polarity Hybridization Carbon Nitrogen Orbital Geometry Molecular Geometry Hybridization of central atom Orbital Geometry O-S-O bond angle Polarity Molecular Geometry Hybridization of central atomi Orbital Geometry Cl-As-Cl bond angles and Polarity
Answer all three please ✓ 2 TABLE 13.2 Inorganic Compounds - Be sure to show the proper geometries! Species Lewis Dot Structure and Resonance structures including formal charge VSEPR Theory and Valence Bond Theory Classifications Molecular Geometry HCN Indicate the hybridization of both the C and N Total Valence Electrons SO₂ Show all reso- nance structures and formal charges. Total Valence Electrons AsCl₂ Are the Cl atoms equato- rial, axial, or both? Total Valence Electrons H-C-N bond angle Polarity Hybridization Carbon Nitrogen Orbital Geometry Molecular Geometry Hybridization of central atom Orbital Geometry O-S-O bond angle Polarity Molecular Geometry Hybridization of central atomi Orbital Geometry Cl-As-Cl bond angles and Polarity
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter8: Advanced Theories Of Covalent Bonding
Section: Chapter Questions
Problem 19E: Strike-anywhere matches contain a layer of KClO3 and a layer of P4S3. The heat produced by the...
Related questions
Question
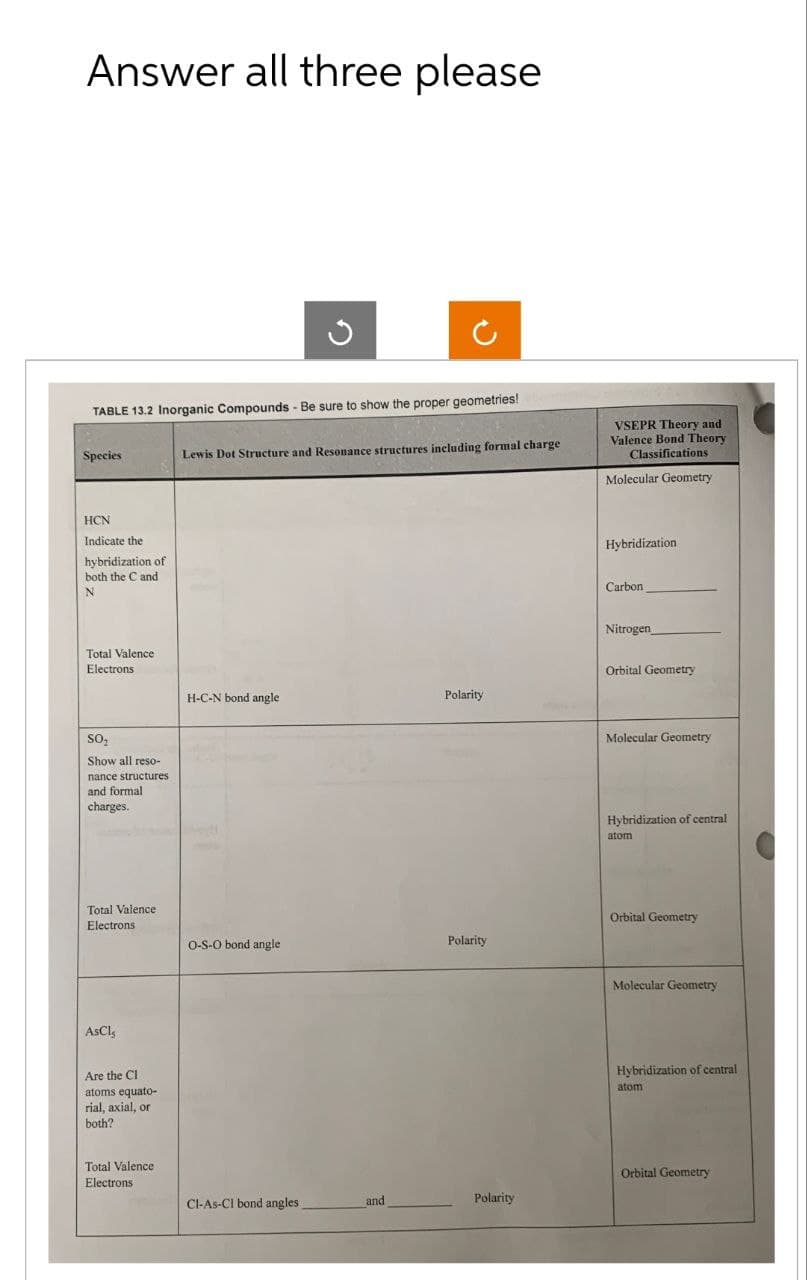
Transcribed Image Text:Answer all three please
✓
2
TABLE 13.2 Inorganic Compounds - Be sure to show the proper geometries!
Species
Lewis Dot Structure and Resonance structures including formal charge
VSEPR Theory and
Valence Bond Theory
Classifications
Molecular Geometry
HCN
Indicate the
hybridization of
both the C and
N
Total Valence
Electrons
SO₂
Show all reso-
nance structures
and formal
charges.
Total Valence
Electrons
AsCl₂
Are the Cl
atoms equato-
rial, axial, or
both?
Total Valence
Electrons
H-C-N bond angle
Polarity
Hybridization
Carbon
Nitrogen
Orbital Geometry
Molecular Geometry
Hybridization of central
atom
Orbital Geometry
O-S-O bond angle
Polarity
Molecular Geometry
Hybridization of central
atomi
Orbital Geometry
Cl-As-Cl bond angles
and
Polarity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning