Chapter18: Electrochemistry
Section: Chapter Questions
Problem 24Q: What is wrong with the following statement: The best concentration cell will consist of the...
Related questions
Question
Answer question 3
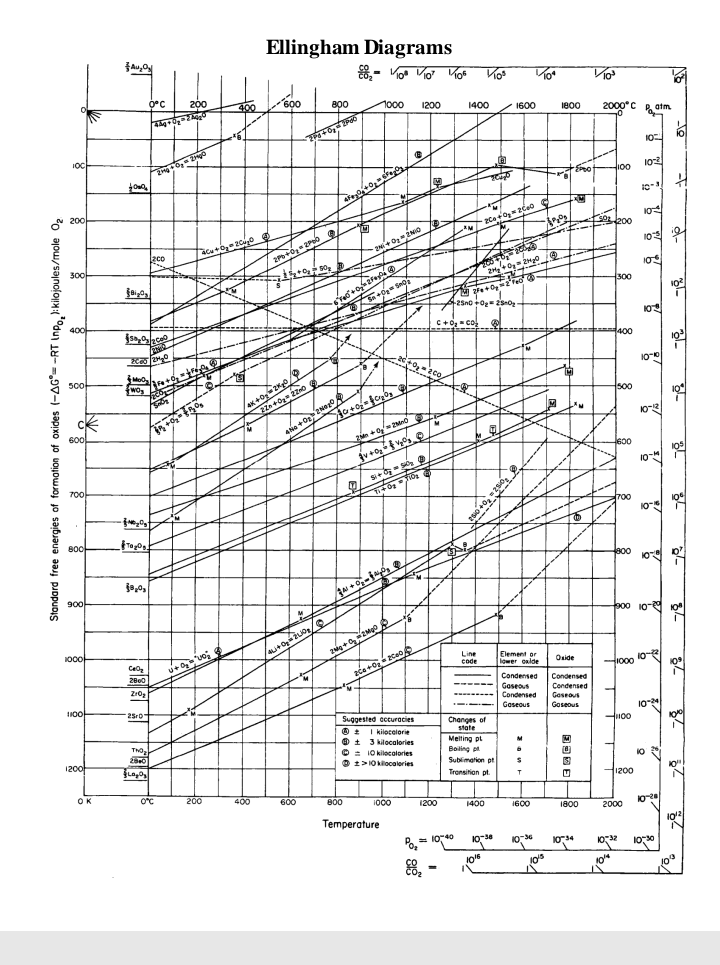
Transcribed Image Text:Ellingham Diagrams
0°C
200
400
600
800
1000
1200
1400
1600
2000*C atm.
ie00
বি
Hi00
10
200
k00
10
2Ni+ Oz2NO
zco
300
ర
300
10
C+0g co
400
100
2co
500
WOS
500
10-
2 +0 2M0
600
I05
-
10
Si+ s
700
700
10
of
800
800
900
900 10- 1O
Line
code
Element or
lower oalde
Oside
HH000 10
2800
Condensed
Condensed
-----m
Goseous
Condensed
Goseous
Zro
Condensed
Goseous
Goseous
25r0
10-
Suggested ccuracies
Changes of
O I kilocelorie
O 1 3 kilocalories
O : IONilecalories
O 1>1Olocaleries
state
Melting pt
Boiling p
10
1200
Sublimation pt
Logo
Tronsition pt.
1200
OK
200
400
600
800
1000
1200
1400
1600
1800
2000
Temperature
E 1040
-30
10
1034
1032
1030
8, -
10"
Standard free energies of formation of oxides (-AG= -RT Inpo, ): kilojoules/mole 02
国可回日
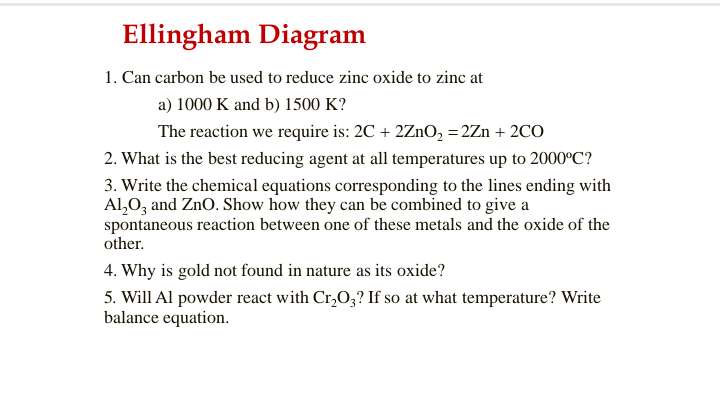
Transcribed Image Text:Ellingham Diagram
1. Can carbon be used to reduce zinc oxide to zinc at
a) 1000 K and b) 1500 K?
The reaction we require is: 2C + 2ZnO, = 2Zn + 2CO
2. What is the best reducing agent at all temperatures up to 2000°C?
3. Write the chemical equations corresponding to the lines ending with
Al,0, and ZnO. Show how they can be combined to give a
spontaneous reaction between one of these metals and the oxide of the
other.
4. Why is gold not found in nature as its oxide?
5. Will Al powder react with Cr,O;? If so at what temperature? Write
balance equation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning