Answer the following question: A student wants to make a 1.600 M aqueous solution of barium sulfate, BaSO4 and has a bottle containing 12.00 g of barium sulfate. What should be the final volume of the solution? Find the numerical answer for this question and make sure to include the following: What is the formula for molarity? What is the molar mass for barium sulfate? When you give your numerical answer, what are the correct significant figures and how do you know that is the correct amount?
Answer the following question: A student wants to make a 1.600 M aqueous solution of barium sulfate, BaSO4 and has a bottle containing 12.00 g of barium sulfate. What should be the final volume of the solution? Find the numerical answer for this question and make sure to include the following: What is the formula for molarity? What is the molar mass for barium sulfate? When you give your numerical answer, what are the correct significant figures and how do you know that is the correct amount?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 33QAP: 33. For each of the following solutions, the number of moles of solute is given, followed by the...
Related questions
Question
When you give your numerical answer, what are the correct significant figures and how do you know that is the correct amount?
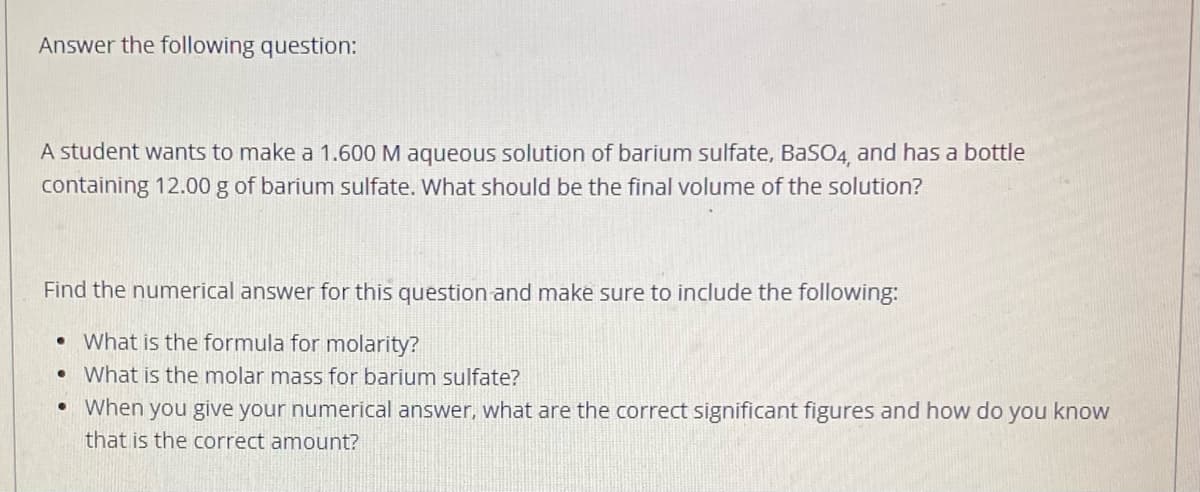
Transcribed Image Text:Answer the following question:
A student wants to make a 1.600 M aqueous solution of barium sulfate, BaSO4, and has a bottle
containing 12.00 g of barium sulfate. What should be the final volume of the solution?
Find the numerical answer for this question and make sure to include the following:
What is the formula for molarity?
What is the molar mass for barium sulfate?
When you give your numerical answer, what are the correct significant figures and how do you know
that is the correct amount?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning