Answer the following questions about the solubility of Ca(OH)2 (Ksp = 1.3 x 106). a. Write a balanced chemical equation for the dissolution of Ca(OH)2(s) in pure water. b. Calculate the molar solubility of Ca(OH)2 in 0.10 M Ca(NO3)2 - c. In the box below, complete a particle representation diagram that includes four water molecules with proper orientation around the Cat ion. 2+ Represent water molecules as Ca2+
Answer the following questions about the solubility of Ca(OH)2 (Ksp = 1.3 x 106). a. Write a balanced chemical equation for the dissolution of Ca(OH)2(s) in pure water. b. Calculate the molar solubility of Ca(OH)2 in 0.10 M Ca(NO3)2 - c. In the box below, complete a particle representation diagram that includes four water molecules with proper orientation around the Cat ion. 2+ Represent water molecules as Ca2+
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter15: Equilibria Of Other Reaction Classes
Section: Chapter Questions
Problem 11E: The Handbook of Chemistry and Physics (http://openstaxcollege.org/l/16Handbook) gives solubilities...
Related questions
Question
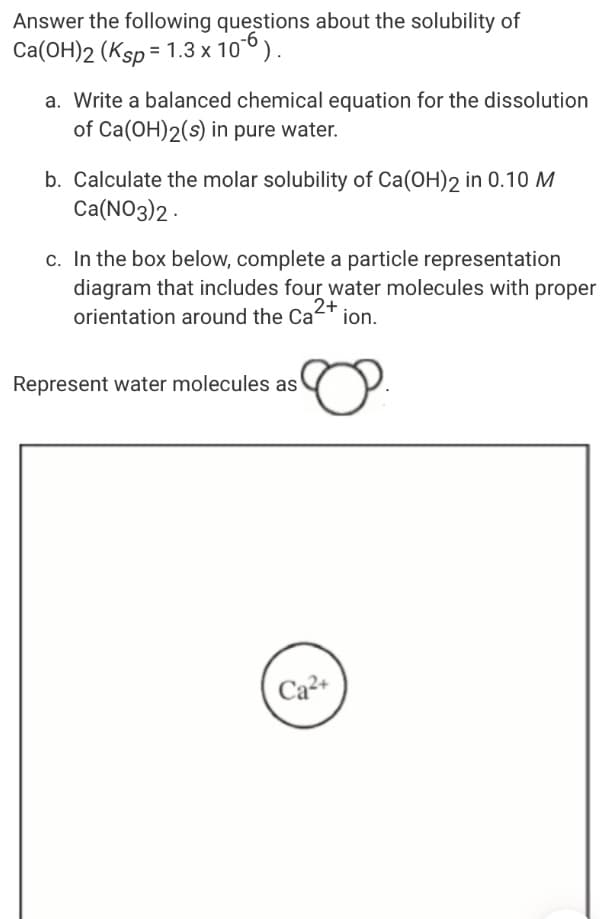
Transcribed Image Text:Answer the following questions about the solubility of
Ca(OH)2 (Ksp = 1.3 x 106).
a. Write a balanced chemical equation for the dissolution
of Ca(OH)2(s) in pure water.
b. Calculate the molar solubility of Ca(OH)2 in 0.10 M
Ca(NO3)2 -
c. In the box below, complete a particle representation
diagram that includes four water molecules with proper
orientation around the Cat ion.
2+
Represent water molecules as
Ca2+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax