Excess amounts of HNO3 and NaOH will be added to separate samples of saturated solution of each salt. Predict what will happen to the solubility of each salt. Determine if solubility of the salt will INCREASE or if the solubility will DECREASE upon addition of HNO3 or NaOH. Leave the space blank if the solubility of the salt is unaffected. Salt Ksp at 25 °C + excess HNO3 + excess Na0H CuCl 1.2 x 10-6 Zn(OH)2 1.2 x 10-17 AgCl 1.8 x 10-10 COCO3 1.4 x 10-13 CaF2 5.3 х 10-9
Excess amounts of HNO3 and NaOH will be added to separate samples of saturated solution of each salt. Predict what will happen to the solubility of each salt. Determine if solubility of the salt will INCREASE or if the solubility will DECREASE upon addition of HNO3 or NaOH. Leave the space blank if the solubility of the salt is unaffected. Salt Ksp at 25 °C + excess HNO3 + excess Na0H CuCl 1.2 x 10-6 Zn(OH)2 1.2 x 10-17 AgCl 1.8 x 10-10 COCO3 1.4 x 10-13 CaF2 5.3 х 10-9
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 52P
Related questions
Question
Clue: Ignore diverse-ion effect and focus on the effect of pH on solubility.
Determine if there are new products that can be formed using the cations and anions of the salt and H+ and OH–?
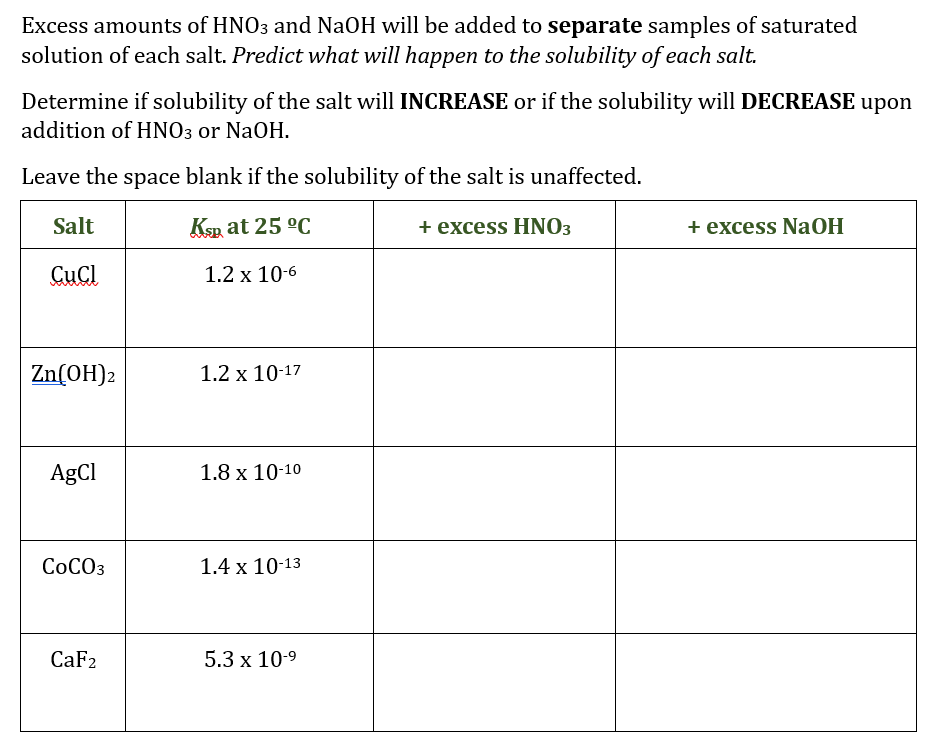
Transcribed Image Text:Excess amounts of HNO3 and NaOH will be added to separate samples of saturated
solution of each salt. Predict what will happen to the solubility of each salt.
Determine if solubility of the salt will INCREASE or if the solubility will DECREASE upon
addition of HNO3 or NaOH.
Leave the space blank if the solubility of the salt is unaffected.
Salt
Ksp at 25 °C
+ excess HNO3
+ excess NaOH
CuCl
1.2 x 10-6
Zn(OH)2
1.2 x 10-17
AgCl
1.8 х 10-10
СОСОЗ
1.4 x 10-13
CaF2
5.3 x 10-9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax