Answer the following questions: Oxidation number of O in K202 Choose... The total oxidation state of all atoms in a neutral species Choose... Addition of Hydrogen Choose... An example of redox reaction Choose... The substance which undergoes reduction in a redox reaction Choose.. Oxidation number of Ca in Cas Choose
Answer the following questions: Oxidation number of O in K202 Choose... The total oxidation state of all atoms in a neutral species Choose... Addition of Hydrogen Choose... An example of redox reaction Choose... The substance which undergoes reduction in a redox reaction Choose.. Oxidation number of Ca in Cas Choose
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter12: The Alkaline Earths And The Halongens-two Families In The Periodic Table
Section: Chapter Questions
Problem 2ASA: Substances A2, B2, and C2 can all act as oxidizing agents. In solution, A2 is green, B2 is yellow,...
Related questions
Question
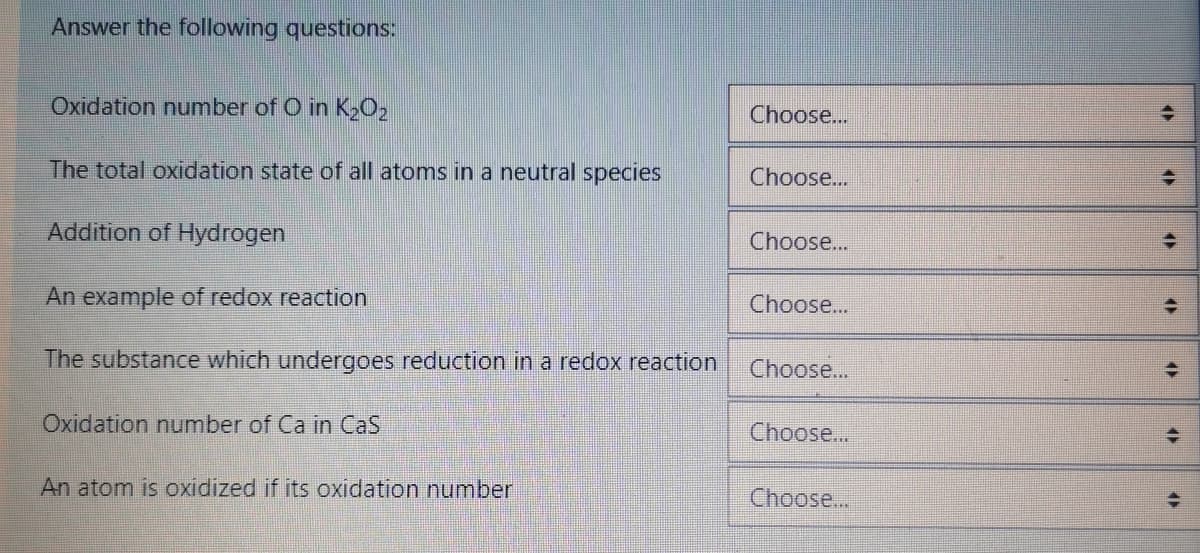
Transcribed Image Text:Answer the following questions:
Oxidation number of O in K2O2
Choose...
The total oxidation state of all atoms in a neutral species
Choose...
Addition of Hydrogen
Choose...
An example of redox reaction
Choose...
The substance which undergoes reduction in a redox reaction
Choose...
Oxidation number of Ca in CaS
Choose...
An atom is oxidized if its oxidation number
Choose...

Transcribed Image Text:Reducing Agent
CdC12 + 2KOH → Cd(OH)2 + 2KCI
-1
0.
Decreases
Oxidation
-2
BaCl2 + NA2SO4 – BaSO4 + 2NACI
increases
CACO3 Ca0 + CO2
+2
Fe203 +2AI– A1203 + 2Fe
Oxidising Agent
Positive
Reduction
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning