Applied load on the steel ball: Diameter of the steel ball: Pb (gram) 10 1 9.8 9.6 Hardness (BHN) 0,9 0,8 9.4 % Radius difference Pb and Sn Give the density of 12% Sn in Pb Alloy 1 0,7 0,6 0,5 0,4 0,3 0,2 0,1 0 Composition 0 Sn (gram) 10 9.8 9.6 9.4 Total (gram) 20 2 19.6 19.2 18.8 Solid Solution Strengthening Tabulated Results kg 51.35 6.35 % Sn 0 2 4 6 mm d₁ (mm) 3.25 2.78 2.40 2.40 % Sn =>> d₂ (mm) 3.30 2.84 2.44 2.40 13.70% Hardness d₁ (mm) 3.52 2.74 2.60 2.56 davrange 10 3.36 2.79 2.48 2.45 10.63 g/cm^3 12 BHN 5.35 7.97 10.21 10.47 14 Answer the following questions Determine the line of best fit in terms of y=mx+c and use the data obtained to predict the hardness of a 9% Sn and Pb alloy.
Applied load on the steel ball: Diameter of the steel ball: Pb (gram) 10 1 9.8 9.6 Hardness (BHN) 0,9 0,8 9.4 % Radius difference Pb and Sn Give the density of 12% Sn in Pb Alloy 1 0,7 0,6 0,5 0,4 0,3 0,2 0,1 0 Composition 0 Sn (gram) 10 9.8 9.6 9.4 Total (gram) 20 2 19.6 19.2 18.8 Solid Solution Strengthening Tabulated Results kg 51.35 6.35 % Sn 0 2 4 6 mm d₁ (mm) 3.25 2.78 2.40 2.40 % Sn =>> d₂ (mm) 3.30 2.84 2.44 2.40 13.70% Hardness d₁ (mm) 3.52 2.74 2.60 2.56 davrange 10 3.36 2.79 2.48 2.45 10.63 g/cm^3 12 BHN 5.35 7.97 10.21 10.47 14 Answer the following questions Determine the line of best fit in terms of y=mx+c and use the data obtained to predict the hardness of a 9% Sn and Pb alloy.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Please assist me with number 1 and 2
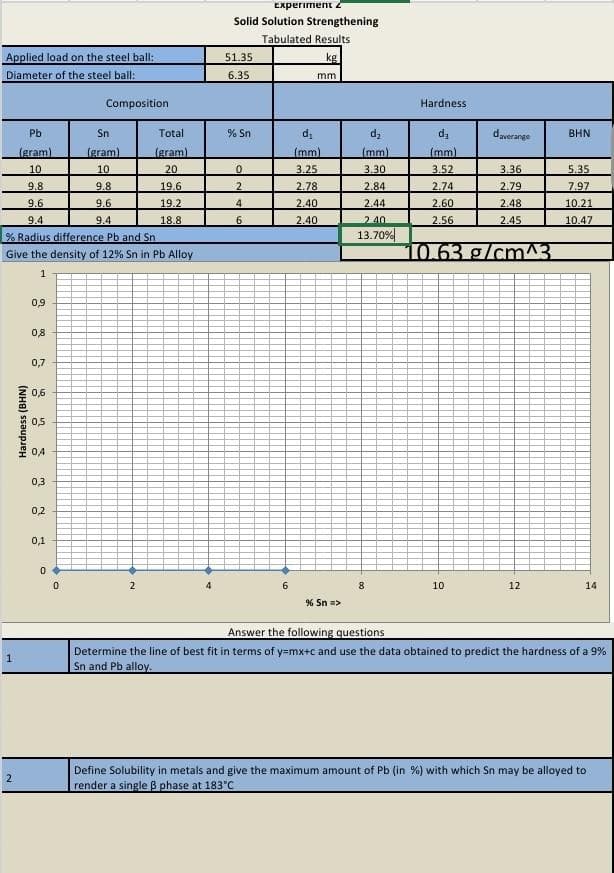
Transcribed Image Text:Applied load on the steel ball:
Diameter of the steel ball:
Pb
Sn
(gram)
(gram)
10
10
9.8
9.8
9.6
9.6
9.4
9.4
% Radius difference Pb and Sn
Give the density of 12% Sn in Pb Alloy
1
1
2
Hardness (BHN)
0,9
0,8
0,7
0,6
0,5
0,4
0,3
0,2
0,1
0
Composition
0
2
Total
(gram)
20
19.6
19.2
18.8
A
Experiment 2
Solid Solution Strengthening
Tabulated Results
kg
51.35
6.35
% Sn
0
2
4
6
6
mm
d₁
(mm)
3.25
2.78
2.40
2.40
% Sn =>
d₂
(mm)
3.30
2.84
2.44
2.40
13.70%
DO
Hardness
d₂
(mm)
3.52
2.74
2.60
2.56
daverange
10
3.36
2.79
2.48
2.45
10.63 g/cm^3
12
BHN
5.35
7.97
10.21
10.47
14
Answer the following questions
Determine the line of best fit in terms of y=mx+c and use the data obtained to predict the hardness of a 9%
Sn and Pb alloy.
Define Solubility in metals and give the maximum amount of Pb (in %) with which Sn may be alloyed to
render a single ß phase at 183°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The