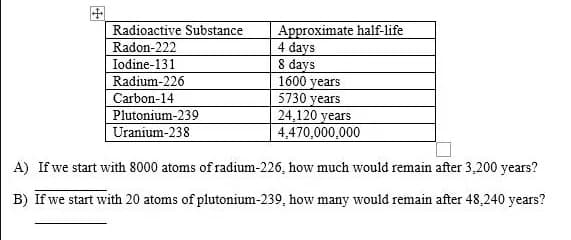
Approximate half-life 4 days 8 days 1600 years 5730 years 24,120 years 4,470,000,000 Radioactive Substance Radon-222 Iodine-131 Radium-226 Carbon-14 Plutonium-239 Uranium-238 A) If we start with 8000 atoms of radium-226, how much would remain after 3,200 years? B) If we start with 20 atoms of plutonium-239, how many would remain after 48,240 years?
Approximate half-life 4 days 8 days 1600 years 5730 years 24,120 years 4,470,000,000 Radioactive Substance Radon-222 Iodine-131 Radium-226 Carbon-14 Plutonium-239 Uranium-238 A) If we start with 8000 atoms of radium-226, how much would remain after 3,200 years? B) If we start with 20 atoms of plutonium-239, how many would remain after 48,240 years?
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter24: Nuclear Chemistry
Section: Chapter Questions
Problem 82A
Related questions
Question
100%
Transcribed Image Text:Approximate half-life
4 days
8 days
1600 years
5730 years
24,120 years
4,470,000,000
Radioactive Substance
Radon-222
Iodine-131
Radium-226
Carbon-14
Plutonium-239
Uranium-238
A) If we start with 8000 atoms of radium-226, how much would remain after 3,200 years?
B) If we start with 20 atoms of plutonium-239, how many would remain after 48,240 years?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning