Use the table on the right to answer the following questions. 11. How long is a half-life for carbon-14? Redioactive Dacay of Carbon-14 100 75% Amount 12. If only 25% of the carbon-14 remains, how old is the material containing the carbon-14? carben 14 50% 25%- 12.5%- 13. If a sample originally had 120 atoms of carbon-14, how many atoms will remain after 16,110 years? 5730 Tirm y 14. If a sample known to be about 10,740 years old has 400 carbon-14 atoms, how many atoms were in the sample when the organism died?
Use the table on the right to answer the following questions. 11. How long is a half-life for carbon-14? Redioactive Dacay of Carbon-14 100 75% Amount 12. If only 25% of the carbon-14 remains, how old is the material containing the carbon-14? carben 14 50% 25%- 12.5%- 13. If a sample originally had 120 atoms of carbon-14, how many atoms will remain after 16,110 years? 5730 Tirm y 14. If a sample known to be about 10,740 years old has 400 carbon-14 atoms, how many atoms were in the sample when the organism died?
Chapter10: Radioactivity And Nuclear Processes
Section: Chapter Questions
Problem 10.51E
Related questions
Question
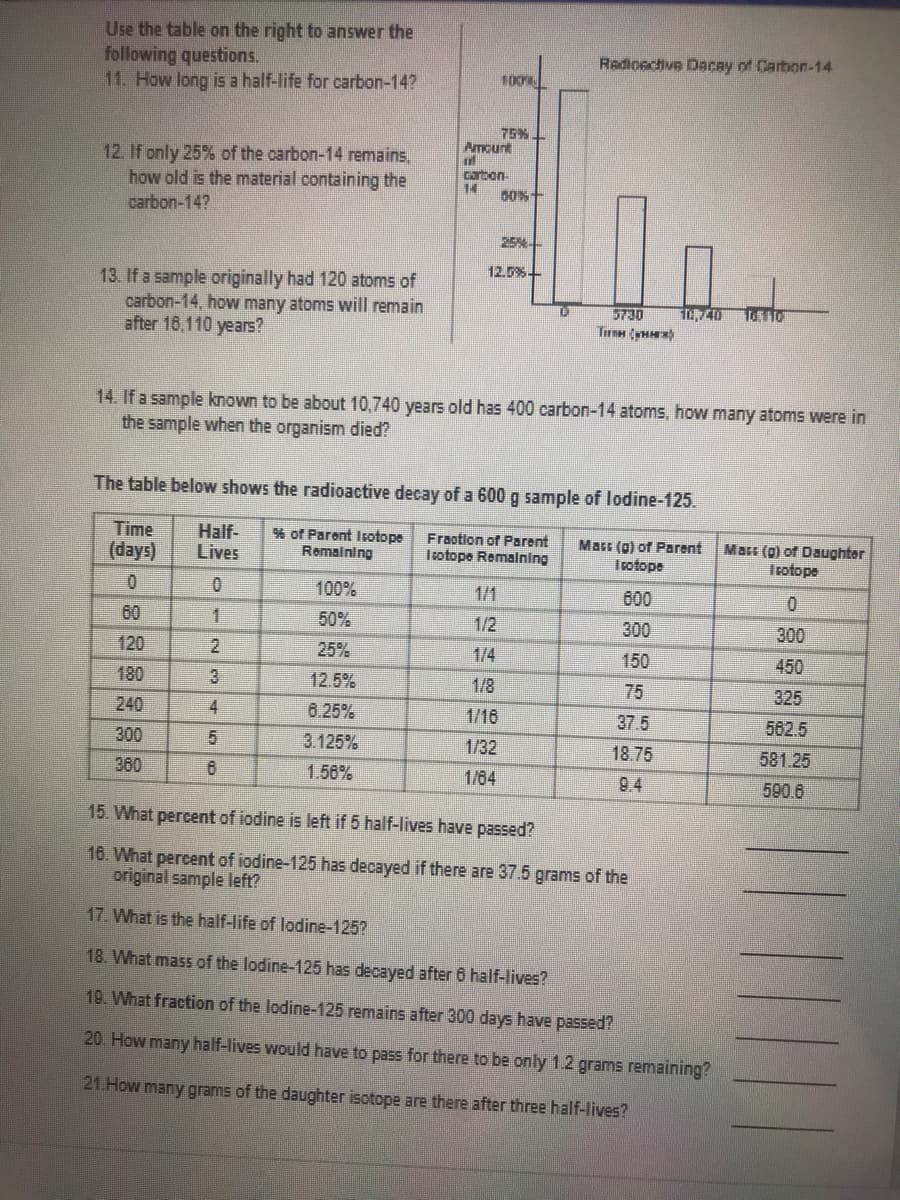
Transcribed Image Text:Use the table on the right to answer the
following questions.
11. How long is a half-life for carbon-14?
Redioective Dacay of Carbon-14
100
75%
Amount
12. If only 25% of the carbon-14 remains,
how old is the material containing the
carbon-14?
carbon
14
50%+
25%
12.5%-
13. If a sample originally had 120 atoms of
carbon-14, how many atoms will remain
after 16,110 years?
5730
Tirm yH)
14. If a sample known to be about 10,740 years old has 400 carbon-14 atoms, how many atoms were in
the sample when the organism died?
The table below shows the radioactive decay of a 600 g sample of lodine-125.
Time
(days)
Half-
Lives
% of Parent Isotope
Remalning
Fraotion of Parent
Trotope Remalning
Mars (9) of Parent
Irotope
Mars (0) of Daughter
Irotope
100%
1/1
600
0.
60
1.
50%
1/2
300
300
120
2.
25%
1/4
150
450
180
3
12.5%
1/8
75
325
240
4
8.25%
1/16
37.5
562.5
300
3.125%
1/32
18.75
581.25
380
6.
1.56%
1/64
9.4
590.6
15. What percent of iodine is left if 5 half-lives have passed?
16. What percent of iodine-125 has decayed if there are 37.5 grams of the
original sample left?
17. What is the half-life of lodine-125?
18. What mass of the lodine-125 has decayed after 6 half-lives?
19. What fraction of the lodine-125 remains after 300 days have passed?
20. How many half-lives would have to pass for there to be only 1.2 grams remaining?
21.How many grams of the daughter isotope are there after three half-lives?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co