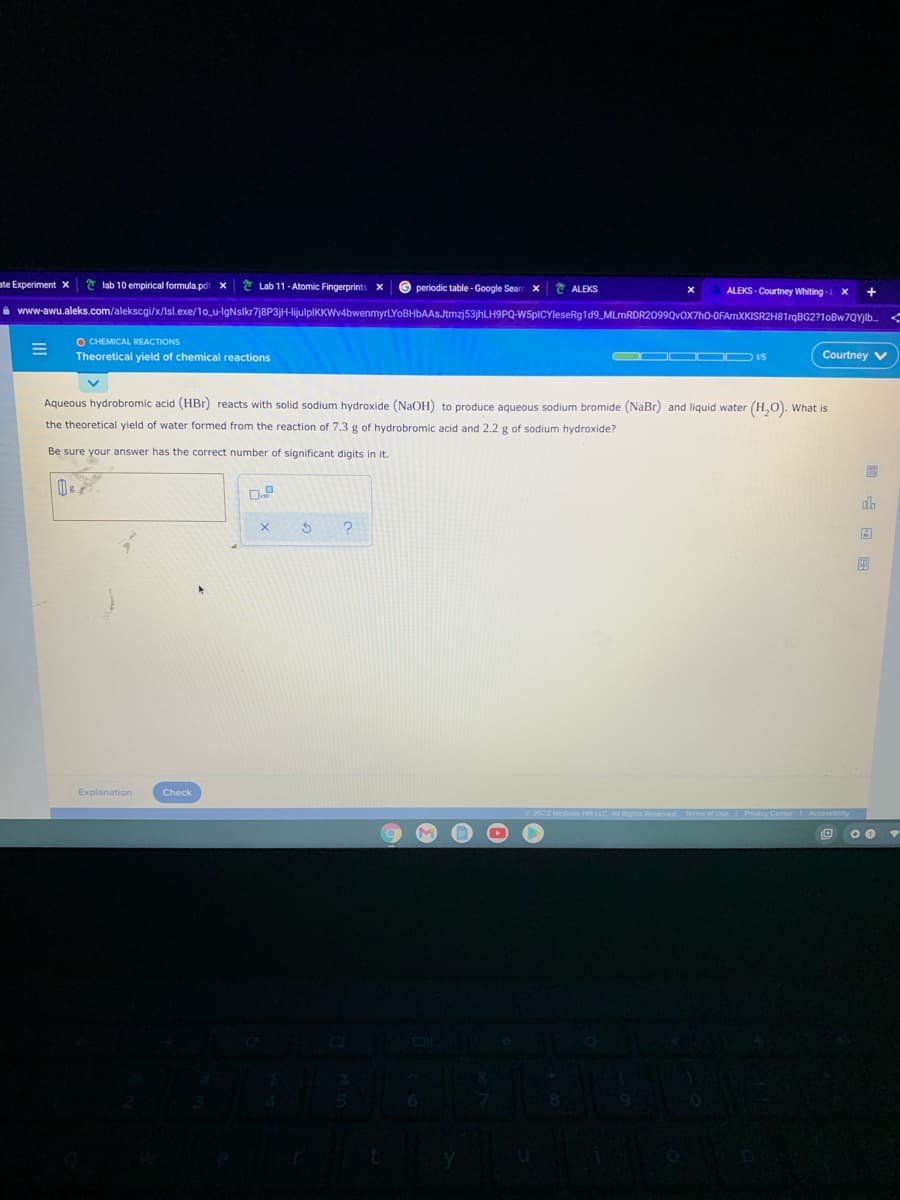
Aqueous hydrobromic acid (HBr) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H,O). What is the theoretical yield of water formed from the reaction of 7.3 g of hydrobromic acid and 2.2 g of sodium hydroxide? Be sure your answer has the correct number of significant digits in it.
Aqueous hydrobromic acid (HBr) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H,O). What is the theoretical yield of water formed from the reaction of 7.3 g of hydrobromic acid and 2.2 g of sodium hydroxide? Be sure your answer has the correct number of significant digits in it.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 38E: An element X bas five major isotopes, which are listed below along with their abundances. What is...
Related questions
Question
Aqueous hydrobromic acid (HBr) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H,O). What is
the theoretical yield of water formed from the reaction of 7.3 g of hydrobromic acid and 2.2 g of sodium hydroxide?
Be sure your answer has the correct number of significant digits in it.
Transcribed Image Text:ate Experiment x
* lab 10 empirical formula.pdt x
* Lab 11- Atomic Fingerprints
© periodic table-Google Searc x
* ALEKS
ALEKS - Courtney Whiting
i www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lijulplKKWv4bwenmyrLYoBHbAASJtmzj53jhLH9PQ-W5pICYleseRg1d9_MLrnRDR2099Qv0X7hO-0FAmXKISR2H81rqBG2?10BW7QYJI..
O CHEMICAL REACTIONS
Theoretical yield
chemical reactions
Courtney V
Aqueous hydrobromic acid (HBr) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H,0). What is
the theoretical yield of water formed from the reaction of 7.3 g of hydrobromic acid and 2.2 g of sodium hydroxide?
Be sure your answer has the correct number of significant digits in it.
alb
Explanation
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
