Archimedes' principle can be used to calculate the density of a fluid as well as that of a solid. Suppose a chunk of iron with a mass of 435.0 g in air is found to have an apparent mass of 397.1 g when completely submerged in an unknown liquid. (a) What mass (in g) of fluid does the iron displace? (b) What is the volume of iron (in cm3), using its density as given in this table? (c) Calculate the fluid's density and identify it. water ethyl alcohol petrol glycerin olive oil
Archimedes' principle can be used to calculate the density of a fluid as well as that of a solid. Suppose a chunk of iron with a mass of 435.0 g in air is found to have an apparent mass of 397.1 g when completely submerged in an unknown liquid. (a) What mass (in g) of fluid does the iron displace? (b) What is the volume of iron (in cm3), using its density as given in this table? (c) Calculate the fluid's density and identify it. water ethyl alcohol petrol glycerin olive oil
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter13: Temperature, Kinetic Theory, And The Gas Laws
Section: Chapter Questions
Problem 20CQ: Because humidity depends only on water's vapor pressure and temperature, are the saturation vapor...
Related questions
Question
Archimedes' principle can be used to calculate the density of a fluid as well as that of a solid. Suppose a chunk of iron with a mass of 435.0 g in air is found to have an apparent mass of 397.1 g when completely submerged in an unknown liquid.
(a)
What mass (in g) of fluid does the iron displace?
(b)
What is the volume of iron (in cm3), using its density as given in this table?
(c)
Calculate the fluid's density and identify it.
- water
- ethyl alcohol
- petrol
- glycerin
- olive oil
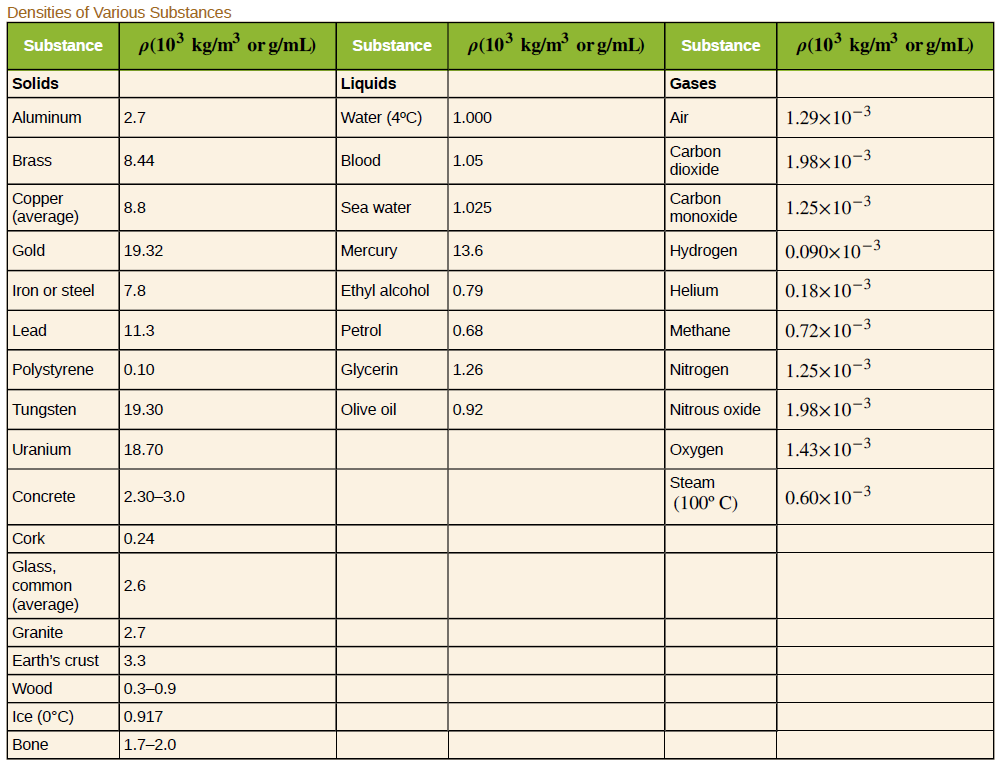
Transcribed Image Text:Densities of Various Substances
p(103 kg/m³ or g/mL)
p(10³ kg/m³ or g/mL)
p(10³ kg/m³ or g/mL)
Substance
Substance
Substance
Solids
Liquids
Gases
Water (4°C)
1.29×10-3
Aluminum
2.7
1.000
Air
Carbon
dioxide
Brass
8.44
Blood
1.05
1.98×10-3
Copper
(average)
Carbon
monoxide
1.25×10-3
8.8
Sea water
1.025
Gold
Hydrogen
0.090x10-3
19.32
Mercury
13.6
Iron or steel
7.8
Ethyl alcohol
0.79
Helium
0.18×10-3
Lead
Petrol
Methane
0.72×10–3
11.3
0.68
Polystyrene
0.10
Glycerin
1.26
Nitrogen
1.25×10-3
Tungsten
19.30
Olive oil
0.92
Nitrous oxide
1.98×10-3
Uranium
18.70
Охудеn
1.43×10-3
Steam
Concrete
0.60x10-3
2.30-3.0
(100° C)
Cork
0.24
Glass,
common
2.6
(average)
Granite
2.7
Earth's crust
3.3
Wood
0.3-0.9
Ice (0°C)
0.917
Bone
1.7-2.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College
