Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 123CP: The early alchemists used to do an experiment in which water was boiled for several days in a sealed...
Related questions
Question
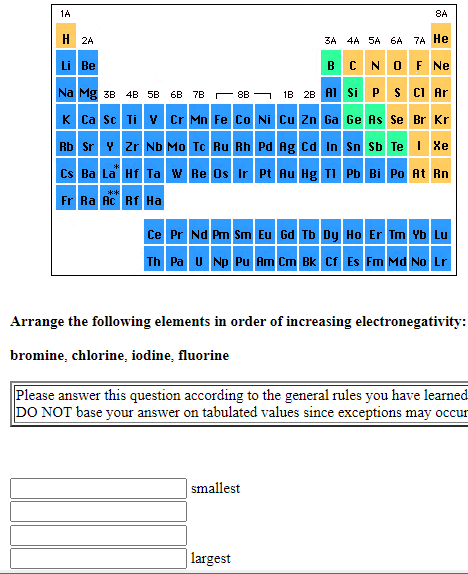
Transcribed Image Text:1A
8A
H 2A
3A 4A SA 6A 7A He
BCNO F Ne
Li Be
Na Mg 3B 4B 5B 6B 7B - 8B – 18 28 A1 Si P S CI Ar
K Ca sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Y zr Nb Mo Tc Ru Rh Pd Ag cd In Sn Sb Te I Xe
Cs Ba La Hf Ta w Re os Ir Pt Au Hg TI Pb Bi Po At Rn
**
Fr Ra Ac Rf Ha
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Arrange the following elements in order of increasing electronegativity:
bromine, chlorine, iodine, fluorine
Please answer this question according to the general rules you have learned
DO NOT base your answer on tabulated values since exceptions may occur
smallest
| largest
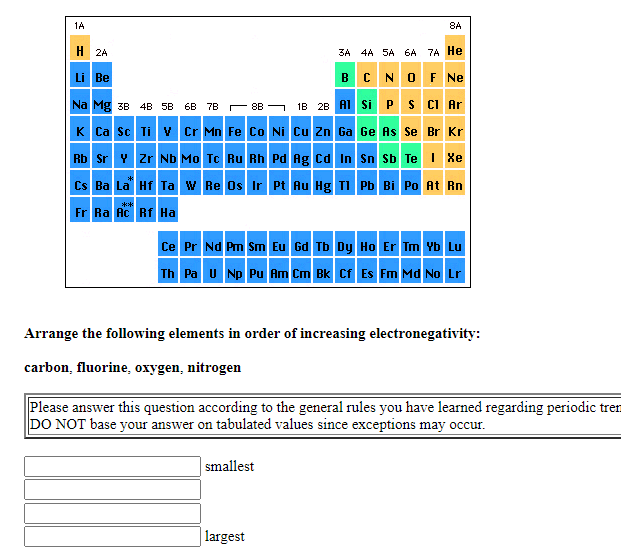
Transcribed Image Text:1A
8A
H 2A
3A 4A 5A 6A
7A He
BC NO F Ne
Li Be
Na Mg 3B 4B 5B 6B 7B r 8B -
1B 2B A1 si PS CI Ar
K Ca Sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Y zr Nb Mo Tc Ru Rh Pd Ag cd In Sn Sb Te I Xe
*
Cs Ba La Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn
**
Fr Ra Ac Rf Ha
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa u Np Pu Am Cm Bk Cr Es Fm Md No Lr
Arrange the following elements in order of increasing electronegativity:
carbon, fluorine, oxygen, nitrogen
Please answer this question according to the general rules you have learned regarding periodic tren
DO NOT base your answer on tabulated values since exceptions may occur.
smallest
largest
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning