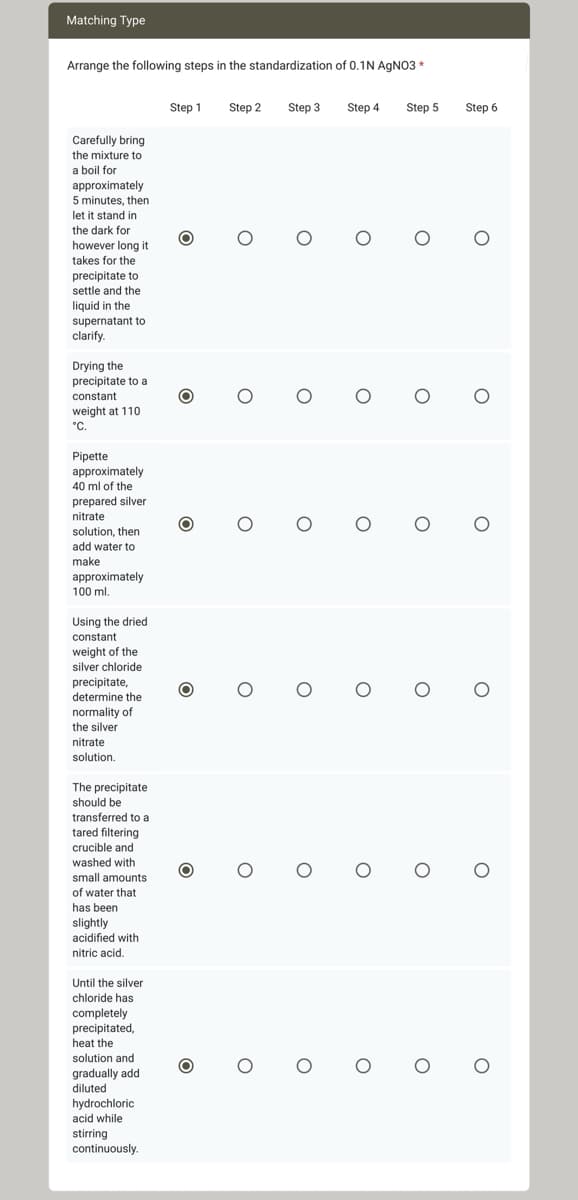
Arrange the following steps in the standardization of 0.1N AgNO3 * Carefully bring the mixture to a boil for approximately 5 minutes, then let it stand in the dark for however long it takes for the precipitate to settle and the liquid in the supernatant to clarify. Drying the precipitate to a constant weight at 110. °C. Pipette approximately 40 ml of the prepared silver nitrate solution, then add water to make approximately 100 ml. Using the dried. constant weight of the silver chloride precipitate, determine the normality of the silver nitrate solution. The precipitate should be transferred to a tared filtering crucible and washed with small amounts of water that has been slightly acidified with nitric acid. Until the silver chloride has completely precipitated, heat the solution and gradually add diluted hydrochloric acid while stirring continuously. Step 1 O Step 2 O O O O O Step 3 O O O O O Step 4 O Step 5 o o O O 0 0 O O O O 0 0 Step 6 O O O O
Arrange the following steps in the standardization of 0.1N AgNO3 * Carefully bring the mixture to a boil for approximately 5 minutes, then let it stand in the dark for however long it takes for the precipitate to settle and the liquid in the supernatant to clarify. Drying the precipitate to a constant weight at 110. °C. Pipette approximately 40 ml of the prepared silver nitrate solution, then add water to make approximately 100 ml. Using the dried. constant weight of the silver chloride precipitate, determine the normality of the silver nitrate solution. The precipitate should be transferred to a tared filtering crucible and washed with small amounts of water that has been slightly acidified with nitric acid. Until the silver chloride has completely precipitated, heat the solution and gradually add diluted hydrochloric acid while stirring continuously. Step 1 O Step 2 O O O O O Step 3 O O O O O Step 4 O Step 5 o o O O 0 0 O O O O 0 0 Step 6 O O O O
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
Transcribed Image Text:Matching Type
Arrange the following steps in the standardization of 0.1N AgNO3 *
Carefully bring
the mixture to
a boil for
approximately
5 minutes, then
it stand in
the dark for
let
however long it
takes for the
precipitate to
settle and the
liquid in the
supernatant to
clarify.
Drying the
precipitate to a
constant
weight at 110
°C.
Pipette
approximately
40 ml of the
prepared silver
nitrate
solution, then
add water to
make
approximately
100 ml.
Using the dried
constant
weight of the
silver chloride
precipitate,
determine the
normality of
the silver
nitrate
solution.
The precipitate
should be
transferred to a
tared filtering
crucible and
washed with
small amounts
of water that
has been
slightly
acidified with
nitric acid.
Until the silver
chloride has
completely
precipitated,
heat the
solution and
gradually add
diluted
hydrochloric
acid while
stirring
continuously.
Step 1
O
O
Step 2
O
O
O
Step 3
Step 4 Step 5
o o
O
O
O
O
O
O
O
Step 6
O
O
O
O
ο ο ο
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning